Home ~ Catalog ~ Links
Dr. Gustave Le Bon
The Evolution of Forces
The International Scientific Series
D. Appleton and Company ~ New York ~ 1908
Part II
The Problems of Physics
Book I
The Dematerialization of Matter and the Problems of
Electricity
Chapter I ~ The Genesis of Current Ideas on Relations
of Electricity & Matter
1. Part of Electricity in Transformation of Chemical Compounds
2. The Like in Dissolution of Simple Bodies
Chapter II ~ The Transformation of Matter Into
Electricity
1. Transformation of Matter into Energy
2. Electrification by Influence
3. Different Forms of Electric Influence
4. Mechanism of Leak from Insulating Bodies
5. Difference of Tension Between Electricity Produced by Chemical
Changes & by Friction explained
Chapter III ~ The Problems of Magnetism, Magnetic
Induction & Lines of Force
1. Problem of Magnetism
2. Problem of Origin of Lines of Force
Chapter IV ~ The Electric Waves
1. Properties of Electric Waves
2. Sensitiveness of Matter to Electric Waves
3. Propagation of Electric Waves to a Distance & Their Utilization
Chapter V ~ The Transparency of Matter to Electric
Waves
1. History of Experiments on Transparency
2. Transparency of Dielectrics to Same
Chapter VI ~ The Different Forms of Electricity
& Their Origin
1. Does Electricity Exist in Matter?
2. Various Forms of Electricity
Book II
The Problems of Heat and Light
Chapter I ~ The Problems of Heat
1. Old and New Ideas on the Causes of Heat
2. Changes of State Under Heat & Energy Resulting Therefrom
3. Can Heat be the Measure of all Forms of Energy?
4. The Conception of the Absolute Zero
Chapter II ~ Transformation of Material Movements
into Ethereal Vibrations & Radiant Heat
1. Nature of Radiant Heat & Transformation by Matter of Ethereal
Vibrations
2. Permanence of Radiation of Matter
3. Electric Emissions which Accompany Heat
Chapter III ~ Transformation of Matter into Light
1. Emission of Light by Matter
2. Influence of Wavelength & Amplitude on Light
3. The Invisible Spectrum
4. Distribution of Energy Throughout Spectrum
5. Absorption of Light by Matter
6. Chemical and Photographic Action of Light
Chapter IV ~ The Dematerialization of Matter by
Light
1. Dissociation of Matter by Different Radiation of Solar Spectrum
2. Origin of Phenomena Exhibited by Radium
Book III
The Problems of Phosphorescence
Chapter I ~ Phosphorescence Produced by Light
1. Different Forms of Phosphorescence
2. Action of Different Parts of Spectrum on Phosphorescent Bodies
3. Phosphorescence of Diamond
4. Intensity of Phosphorescence & Temperature
5. Decay of Phosphorescence by Action of Time
Chapter II ~ Phosphorescence Produced by Heat
1. Method of Observation
2. Properties of Bodies Phosphorescing by Heat
3. Analogies between Phosphorescence by Light & Heat
Chapter III ~ Phosphorescence from Other Causes
than Above
1. Phosphorescence by Impact & Friction
2. By X and Cathode Rays & High-Frequency Effluves
3. By Chemical Reactions
4. Phosphorescence of Living Beings
5. Of Gases
Chapter IV ~ The Causes of Phosphorescence
1. Phosphorescence as a Manifestation of Intra-Atomic Energy
2. Chemical Reactions Causing Phosphorescence
Book IV
Black Light
Chapter I ~ Invisible Phosphorescence
1. Divisions of Black Light
2. History of Invisible Phosphorescence
3. Properties of Invisible Phosphorescence
4. Transformation of Invisible Phosphorescence into Visible
5. Invisible Phosphorescence Preceding Visible
6. Comparative Effects of Infrared Rays and Heat on Phosphorescence
7. Radiations of Metals and Non-Phosphorescent Bodies
Chapter II ~ The Infrared Rays & Photography
Through Opaque Bodies
1. Visibility through Opaque Bodies
2. Photography through Same
3. Instantaneous Photography in Dark
4. Transparency of Different Bodies to Infrared Rays
5. Use of Invisible Rays to Make Distant Bodies Visible
Chapter III ~ The Part Played by the Various Luminous
Radiations in Vital Phenomena
1. The Part of Light in Vital Phenomena
2. Observation of Effects of Solar Spectrum in Plant Life
3. New Method of Study of Physiological Action of Infrared Rays
Chapter IV ~ The Antagonistic Properties of Some
Regions of the Spectrum
1. Rays Which Illuminate & Rays Which Extinguish
2. Opposite Properties of Different Regions of the Spectrum
Book V
Forces of Unknown Origin & Hidden Forces
Chapter I ~ Universal Gravitation & Hidden
Forces
1. Causes of Gravitation
2. Consequences of Gravitation
3. Forces Dimly Seen
Chapter II ~ The Molecular & Intra-Atomic
Forces
1. Attractions and Repulsions of Material Elements
2. Molecular Equilibria
3. The Force and the Form
Chapter III ~ The Forces Manifested by Living
Beings
1. Living Matter and Cellular Life
2. Instability the Condition of Life & Intra-Atomic Energies
3. Forces Which Regulate the Organism
4. Morphogenic Forces
5. Interpretation of Vital Phenomena
Index of Subjects [Not included]
Index of Names [Not included]
Part II
The Problems of Physics
Book I
The Dematerialization of Matter and the Problems of
Electricity
Genesis of the Current Ideas on the Relations of Electricity with Matter
The first part of this work was devoted to the development of the theories deduced from my earlier experiments. This second part will be especially experimental. My observations of the dissociation of matter led me into very varied researches. Notwithstanding their fragmentary nature, they may, I think, interest the reader; and the account of them will be given in such a way as not to interfere with the general plan of this work.
1. The Part Played by Electricity in the Transformation of Chemical Compounds ~
The laws of physical phenomena may be determined, but as we are ignorant of their underlying causes, their interpretation necessarily varies. If the facts do not change, the explanation of them is frequently modified. A great theory once accepted is immediately applied to the interpretation of known facts. The doctrine of the conservation of energy has for 50 years seemed able to furnish the key to all phenomena. It is now the theory of electrons for which this role appears to be reserved. By reason of the importance now given to the prevailing ideas on the atomic structure of electricity, it may be interesting to point out their genesis.
Now dogmas are spontaneous only in appearance. Those who are attracted by a new belief especially appreciate its novelty, while it is in reality very old. Its history, generally forgotten by the textbooks, shows how certain ideas grow up and the slow progress of their evolution.
It is to the illustrious Davy, about the beginning of the last century, that the origin of the present ideas on electrical dissociation, now termed ionization, goes back. Having passes a current through a solution of potash, and having noticed that the potassium went to one of the poles and the oxygen to the other, he concluded, rightly but much in advance of his time, that the two elements of a compound are charged with different electricities, which are neutralized by combination. The affinity which draws together and associates the elements of bodies must have its origin in the attraction of contrary electricities. We do not state the matter otherwise at the present day.
It was on the hypothesis of Davy that Berzelius founded the dualistic theory which governed chemistry for 30 years. The compounds called binary, such as acids and oxides, were formed from an electro-negative united to an electro-positive element by the attraction of their contrary electricities. The compounds called tertiary -- that is to say, salts -- came from the combination of an electro-positive base and an electro-negative acid.
The dualistic theory vanished when Dumas. Laurent, and Gerhard discovered the phenomenon of substitutions, and showed that in a compound an electronegative element could be replaced by an electropositive without sensibly changing its properties. Trichloroacetic acid, for example, is acetic acid in which three atoms of chlorine (an electronegative element) have replaced three atoms of hydrogen (an electropositive one). The conceptions of Davy and Berzelius, correct as they were, were abandoned for a long period.
Their readoption was the consequences of the researches of the illustrious Faraday. Towards 1830, he discovered the laws of electrolysis, and measured the electric charges given up by bodies at the poles of the battery when they are decomposed by the electric current. He noted that when an electric current is passed through the solution of a salt, the latter is decomposed into two elements charged with contrary electricities, which are found at the two poles. This operation, the result of which is to resolve compound bodies into their elements, is called, as we know, electrolysis. The bodies capable of undergoing such an operation are called electrolytes. The products of the decomposition constitute ions. Simple bodies, considered as such to be indecomposable, evidently could not be electrolytes and consequently produce ions. This last was a fundamental point.
After having verified the enormous charge of electricity -- 96,000 coulombs for a gram of hydrogen electrolysis and the equality of this charge for all bodies of equal atomic weight, Faraday recognized, thus confirming the idea of Davy and Berzelius, that chemical phenomena are only electrical phenomena -- that is to say, displacements of electricity.
"The numbers representing the equivalent weights of bodies", he says, "represent simply the quantities of electricity. The electricity determines the equivalent number because it determines the forces of combination. By adopting the atomic theory, we say that the atoms of bodies equivalent to one another in their chemical combination bear the same charges of electricity. It is the quantity of electricity associated with the different atoms which constitutes electrical affinity". The most recent ideas simply represent the adoption and extension of Faraday’s views. At the present day, the theory of ionization reigns without a rival. It has given birth to a new chemical nomenclature in which the properties of bodies are expressed as a function of their electric charges, and no longer, as Berthelot wished, as a function of their thermal properties.
Thermochemistry is now looked upon almost as a doctrine in course of disappearance. We now consider the study of the electrical reactions of bodies as far more important than that of their thermal relation. Charges of electricity and the manner in which they are distributed generate all the properties of bodies and the reactions which their combinations exhibit.
The actual theory of ionization may be summed up in the following statement, which does but repeat exactly the ideas of Faraday: Bodies are composed of elements or ions charges, some with positive, others with negative electricity, and united at first in the neutral state. Under the influence of the battery current, the neutral molecule dissociates into positive and negative elements, which go to the poles of contrary names. The decomposition of a neutral salt may be represented by such an equation as the following: NO3K = NO-3 + K+.
When an ion leaves a solution in order to precipitate itself at an electrode charged with electricity of contrary sign -- by reason of the attraction exercised between two opposite electric charges -- it then receives from the electrode a charge exactly equal but of contrary sign to that which it before possessed. To ascertain this quantity -- to know, for example, the electric charge of the atoms of 1 gram of hydrogen -- we need only measure with suitable instruments the expenditure of electricity necessary to neutralize the ions set at liberty.
At the period of the history of ionization just briefly sketched, it was admitted that the dissociation of the elements of bodies into ions charged with electricity could only be effected under the influence of an electric current, but soon we had to go much further.
Adopting the theoretical ideas put forward by Clausius, Arrhenius recognized that an electric current was in no way necessary to produce the dissociation of compounds into ions. In dilute solutions, the bodies dissolved must be separated into ions by the mere fact of solution. When the electrodes of a battery are plunged into such a solution, the ions must simply be attracted by them -- the positive ions by the negative pole, and the negative ions by the positive pole.
According to this theory, which is evidently very hypothetical, but which has been admitted because it much facilitates explanations, the dilute solution of a metallic salt would contain something quite other than the salt itself. A dilute solution of sea salt, for example, would in no way contain the chloride of sodium we are acquainted with. It would contain chlorine atoms and sodium atoms in a free state.
This chlorine and this sodium in the state of ions must differ much from the substances generally known by those names, since the sodium of our laboratories cannot be introduced into water without decomposing it. The difference must be due to the fact that in the ion chlorine and the ion sodium the electricities are separated, while in the substances known by the same names they are neutralized.
All these theories and the experiments whence they are derived show us that electricity is every day more and more considered as the essential factor in the properties of bodies. It must be solely from their electrical charge that these properties are derived. The chemical activity of an acid and of a base must be the simple consequence of the proportion of ions they contain. A strong acid such as sulfuric acid must contain a great number of free ions, and a feeble one like acetic acid very few. Chemical reactions are now more and more regarded as simple reactions of ions. Atomicity or valency -- that is to say, the aptitude of atoms to unite with a greater or less number of other atoms of various bodies -- must depend on their capacity for saturation with electricity. It is thus, for example. That in a salt like chloride of zinc (ZnCl2) the atom zinc, by reason of the greatness of its positive charge, can hold in equilibrium two negative ions of chlorine.
Such is the current theory. It is very probable that things happen in a less simple, perhaps even in a very different manner; but when an explanation fits in fairly well with known facts, it is wise to be satisfied with it.
The theory of electrons, according to which the electric fluid is composed of an aggregate of particles of definite magnitude, is a direct consequence of the preceding views, and could only give them further precision. This theory, which goes back to Helmholtz, and has been developed by Lorentz, also explains chemical compounds and decompositions by the aptitude of atoms for acquiring and losing electrons. Their degree of valency would thus depend on the number of electrons they might lose or capture. Those which, like the atoms of helium or of argon, must be too stable to acquire or lose any, would be unable to form any combination. All chemical forces would have an electrical origin. Affinity would recognize no other cause. His, again, is exactly what Faraday said and Davy maintained.
2. The Part Played by Electricity in the Dissociation of Simple Bodies ~
The idea that we might separate particles of electricity from their material support was not imaginable before the recent discoveries. It was even so contrary to the early experiments, which seem to show that electricity could not be transported without material support, that for a long time the cathode rays were considered to be formed by the projection of material particles.
This separation of a charge of electricity from its support seemed impossible. Still more impossible did it appear that a simple body could be subjected to ionization -- that is to say, to a separation of elements such as has just been described above. A simple body, being by definition incapable of decomposition, evidently could not be dissociated. To separate the chlorine from the potassium in chloride of potassium was easy; but how could we suppose it possible to extract from chlorine something which was not chlorine and from potassium anything other than potassium.
In less than 10 years, however, that which was considered impossible has ceased to be so. The study of the cathode rays, or of the conductivity of gases, of radioactive emissions, and, finally, my demonstration of the universality of the dissociation of matter, have proved that from simple bodies something quite different from them can be extracted. This something, identical in the most various subjects, being endowed with electrical properties, we will consider as constituted of those particles to which the name of electrons has been given.
Whatever this interpretation be worth, it was certain that simple bodies could also be dissociated; but as this was an idea directly contrary to the doctrines then ruling, and as it was nevertheless necessary to find some theory to explain the observed facts, the old doctrine of ionization was adopted. It was therefore admitted that the electrified particles observed in gases or in dissociated simple bodies were the result of their ionization. The expression, having been accepted long since, could shock no one, while the idea of the dissociation of a simple body would have seemed disquieting. Yet the two words signify exactly the same thing. When we look only at the facts, it is evident that when from the atoms of a simple body something quite different from it has been taken, the atoms of which it is composed have necessarily been dissociated.
It is, moreover, this conclusion which, after many hesitations, has finally been reached. It was the study of radium which carried conviction with it. This body presents in an eminent degree properties which all bodies possess in a very slight degree. It emits in abundance the products of the dissociation of its atoms. By studying these products I recognized their analogy, first with the emissions of particles in Crooke’s tubes, and then with the effluves emitted by all bodies under the action of light or of various influences. Finally, it had to be acknowledged that the dissociation of matter is, as I long ago proved, a universal phenomenon.
All these experiments, many of which showed us particles of electricity freed from their material support, have naturally given great force to the theory of atomic electricity, otherwise called the electronic theory. Having sufficiently set out this in my former work, it would be useless to go back to it here. No objection can be taken to it when it is confined to regarding electricity as composed of discontinuous particles; but there does not seem to be any necessity whatever for considering matter as composed of electrons. Electricity is, like heat and the other forces, one of the forms of intra-atomic energy. From all matter we can extract electricity and heat; but there is no more reason to say that matter is composed of particles of electricity than to assert that it is composed of particles of heat.
It would be as useless, however, to combat the electronic theory at the present day, as it was in Newton’s time to contest the emission hypothesis in optics. Those who attempted it were not even listened to, although the future has shown how right they were. I shall therefore not try to dispute its worth. This task is the less necessary, that it is very easy to express the phenomena in current language. I shall therefore continue to use it for clearness of demonstration.
"The electron", Poincare says rightly, "has conquered physics, and many adore the new idol rather blindly". The idol, in reality of long standing, has only changed its name. To attempt to reduce matter to a single element is indeed an old idea. It translates into fact a mental aspiration and a craving for simplicity with which nature is doubtless not acquainted. One ought not to speak evil of such cravings, for they are the begetters of effort. Thus provisional doctrines and beneficient chimeras stimulate our labors. We unceasingly pursue the Sisyphus task of explanation, but always with the hope that it is for the last time.
The Transformation of Matter into Electricity
1. Transformation of Matter into Electricity ~
Several chapters of my last work were devoted to showing how matter can, by dissociation, return to the ether by a series of successive stages. Among the most constant products of this dissociation electricity is found. It is one of the important manifestations of that liberation of intra-atomic energy which always accompanies the dematerialization of matter.
Since electricity in motion represents energy, it may be said that the transformation of a body into electricity realizes a change of matter into energy. Such a phenomenon being absolutely contrary t the fundamental principles of modern science, my theory will not be acceptable until after a radical conversion of current ideas. It will not therefore be useless to return to so important a subject, and to add new experimental proofs in support of those already set forth. It is equally necessary to show that this doctrine gives the key to phenomena for which either the most insufficient explanations or no explanations at all have hitherto been furnished.
2. Electrification by Influence ~
Electrification by influence, by virtue of which a tick of sealing wax which has been rubbed attracts light bodies such as a suspended ball of pitch, is the most simple of electrical phenomena, and the earliest observed. The usual explanation given of it is too well known for it to be necessary to reproduce it here. It is, moreover, the same as that of the phenomena of the same order which we shall shortly have to deal with, when showing how unacceptable is the classic theory.
This experiment of the attraction of light bodies is in reality one of the most remarkable in physics, and it would have only been necessary to observe it attentively in order to discover the proof of the dissociation of matter and of the existence of intra-atomic energy -- that is to say, of two important principles of modern science.
In the old theories, the various experiments in electricity by influence implied this very singular consequence, that with the limited quantity of electricity existing on a body, we could extract from another body an unlimited quantity of electricity.
Let me recall, to put this essential point clearly in evidence, the classic experiment demonstrating electrification by influence.
A metallic sphere (Figure 1) placed on an insulated support is electrified in one way or another, and thus receives a definite quantity of electricity, the magnitude of which is easily measurable. Let us being near to this sphere at a little distance a conductor -- for example, a long cylinder of metal B, C, placed on an insulator D. We immediately recognize by the ordinary means that the cylinder is charged with electricity. If the ball has in the first instance received positive electricity, it will be observed that the part of the cylinder next to it is charged with negative and its other extremity with positive electricity. If, then, this latter is touched so as to connect it with the earth, the whole cylinder remains charged with negative electricity. This may now be transferred to some other body by moving the cylinder by its insulated support.
Figure 1 ~
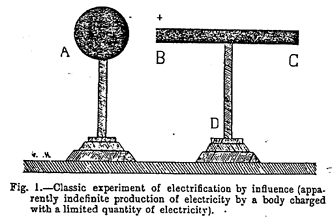
After having discharged the cylinder we have only, in order to recharge it, to bring it again near the ball and act as before. As these successive operations -- charge and discharge of the cylinder -- can be repeated an unlimited number of times, it necessarily follows that, with the limited charge of electricity on an electrified ball, there can be generated on another body an unlimited quantity of electricity.
Physicists, moreover, recognize this clearly. Jamin says in his Cours de Physique de l’Ecole Poklytechnique (4th ed., vol 4, p. 187): "Influence enables us to obtain, by means of a limited quantity of positive electricity an indefinite quantity of negative electricity". We explain this apparent creation of energy by saying that, to charge a body several times by influence it must be displaced -- that is to say, an amount of work must be expended representing the equivalent of the electricity generated at each new operation.
Feeble as is this explanation of the transformation into electricity of the simple movement of translation of a body, it seemed useful to take away every appearance of probability form it by producing an indefinitely extended electrification without any trace of displacement of the body electrified. This is realized in the following experiment, which shows a body isolated in space becoming by influence, a continuous source of electricity.
Figure 2 ~
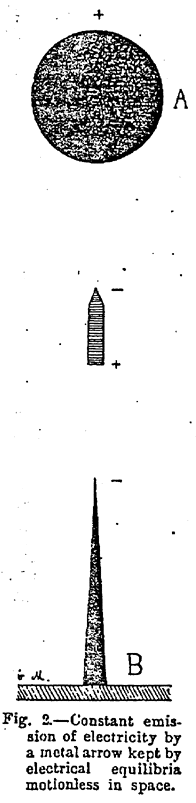
A (Figure 2) is the influencing electrified body. It is kept charged with positive electricity by any means we please (1). Below we see a piece of aluminum or gold leaf half a centimeter wide and two centimeters high. B is a metal rod placed on a table or held in the hand. After a few trials a position is easily found in which the strip of metal remains wholly and indefinitely motionless in space. In spite of its slight consistence it remains fixed and rigid as if held by springs, and its point throws out little sparks visible in the dark. Its various parts take charges, the direction of which is shown in the figure.
[(1) If the ball is charged with negative instead of positive electricity, the aluminum leaf will attach itself to it and will not remain motionless in space.]
This experiment has always impressed those physicists to whom I have shown it, because the equilibrium observed is difficult of explanation. It must not be supposed that the metal arrow is simply immobilized by the electric attractions exercised in contrary direction on its extremities. It would be impossible to maintain in a block of iron between the poles of magnets acting on it in opposite directions. It is only in oriental legends that we see Mahomet’s coffin immobilized in space by the action of magnets placed in its neighborhood.
The aluminum arrow in the above experiment only remains motionless because it is the seat of a constant emission of particles. It is, moreover, not a question of equilibrium we have to examine here, but the continuous emission of electricity by he metal under the influence of the electrified ball. This omission is made plain by the little sparks, easily seen in the dark, which issue from the point of the metal.
In the experiment thus arranged, the electricity produced can only issue from the elements composing the strip of aluminum. How can we explain the fact that from an isolated fragment of metal we can extract an apparently infinite quantity of electricity?
Readers conversant with my previous researches will certainly have guessed the explanation. It is wholly contained in the enunciation of the three following principles: (1) Matter contains an enormous reservoir of energy; (2) it can be dissociated; (3) in dissociation it liberates, in various forms, but especially as electricity, a part of the intra-atomic energy accumulated within it at the moment of its formation.
The metal arrow in the preceding experiment is in every way comparable to a fragment of radium. The only difference is that, instead of dissociating itself spontaneously like the radium, the fragment of aluminum is only dematerialized under the influence of electric action.
When we rub a body, when we place it under the influence of an electrified source, or when we subject it to any sort of disturbance of the ether, such as a luminous ray, we are doing quite a different thing to transforming (as the textbooks teach) movement or any other energy into electricity. We do not thus affect a transformation, but a liberation of force. We simply dissociate matter by bringing suitable reagents to bear upon it. Electricity is one of the manifestations of this dissociation.
The magnitude of intra-atomic energy being, as I have shown, immense, it will be understood that from a very infinitesimal portion of matter there may issue a very large quantity of energy. This emission is considerable, but it is not infinite; and it is probable that if the above experiment were continued for several centuries, we should see the aluminum become gradually less by its conversion into electricity, and finally disappear. We should then have witnessed the complete transformation of matter into energy.
The experiment on electrification by influence described in this chapter realizes this evolution exactly. We might have read it in the elementary facts which have been before the eyes of physicists for several centuries without their understanding their import.
It further results from this experiment that electricity, which is one of the products of the dissociation of matter, is at the same time one of the most active agents of this dissociation. It constitutes by its special attractions for the elements of matter one of those appropriate reagents of which the importance has been shown in a chapter of my earlier. It is one of those against which matter is defenseless, while it is able to strive against very energetic but not appropriate reactions.
The irresistible power of attraction of a minute particle of electricity dissociates matter which the impact of a shell might pulverize and even volatilize, but could not dematerialize.
3. The Different Forms of Electric Influence ~
In the last experiment, that of the strip of aluminum immobilized in space, we have seen influence manifest itself by the projection of luminous particles, while in the classical experiment previously related this projection is not visibly produced.
I was thus led to suspect that the phenomenon of electrification by influence might take place through different mechanisms. Researches made to verify this hypothesis have enabled me to recognize its correctness.
Let us first of all note that by the theory of electrons, electrification by influence would obtain a very simple explanation, since it would be enough to admit that, from bodied subjected to influence there pass visible or invisible electrons, which are immediately transformed into ions by their passage in the air, according to the well-known mechanisms.
M. de Heen has opposed to this interpretation a very grave objection: If electrification by influence were due to projections of particles proceeding from the influencing body, it would be arrested by the interposition of a thick sheet of glass, which is not at all the case. This is plainly seen in the experiments given later on, which show the action of influence upon a body entirely protected by three concentric metallic enclosures. The theory of electrons is therefore quite powerless to explain electrification by influence. It may result from somewhat different mechanisms, as shown in the following experiments, which prove that bodies subjected to the same electric influence may, according to circumstances, acquire charges of contrary sign.
No one is aware -- for this, again, is one of the most elementary experiments in physics -- that if an electrified substance -- a stick of ebonite or glass, for instance -- excited by friction is brought close to the ball of an electroscope, the leaves are charged by influence and immediately diverge. They fall as soon as the electrified body is withdrawn, because the charges of contrary sign produced on the ball and on the leaves recombine as soon as the influence ceases.
Generations of physicists and their pupils have repeated this experiment. Had they thought of prolonging the presence of the rod above the ball of the electroscope for a few minutes instead of a few seconds, they would have observed -- not without some astonishment, perhaps -- that the gold leaves remain separated after the rod has been withdrawn, and that the sign of the charge of the ball has changed -- that is to say, that it has become negative from positive, as it was at first. The effects produced depend, moreover, on the form given to the body influenced, as shown in the subjoined figures (Figures 3 to 5). Here we see modes of influence succeeding, but not superposed on, each other. It is impossible to confuse them, for to each of them there corresponds a different charge of electricity.
Figure 3 / 4 / 5 ~
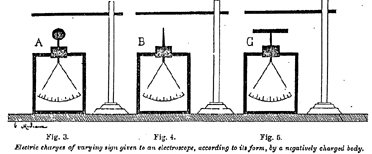
In the case of Figure 5 the plate-like form of the influenced body is the cause that the sign of the charge does not change, and that the leaves fall when the electrified rod is withdrawn.
In the case of Figures 3 and 4 it is quite otherwise; the leaves remain charged when we take away the rod. With the electroscope of Figure 4 the experiment is effected in a few seconds, while it is necessary to continue the presence of the ebonite rod for some 12minutes for it to succeed with the ball electroscope of Figure 3.
The explanation of the maintenance of the charge or of its reversal after the rod of ebonite has been withdrawn is very simple.
Take, for example, the case of Figure 3. The charge of the ball under the influence of the action of the negatively electrified ebonite rod is, as we know, at first positive, and that of the leaves negative. If the ball is touched with the finger, as when we charge an electroscope by influence, the negative charge passes into the earth, while the positive charge, kept back by the negative electricity of the rod, remains on the ball and flows into the leaves as soon as the finger and the electric rod are withdrawn. Finally, the leaves are charged positively.
If the ebonite rod be left a sufficient time near the ball, we note, on the contrary, that when it is withdrawn without touching the ball of the electroscope with the finger, the leaves remain negatively charged instead of positively. Why?
As soon as the ebonite rod is brought close to the ball of the electroscope it becomes positively charged by influence -- as in the experiment with the cylinder related in another paragraph -- and the negative electricity is drawn into the leaves. If, instead of withdrawing the ebonite rod, its presence is prolonged, it strongly attracts the particles of positive electricity from the ball, and when it is withdrawn the gold leaves keep the negative electricity they took at first, which is then spread over the ball. Therefore this latter, which was first charged positively, now becomes charged negatively. Finally, the whole instrument possesses a charge of negative electricity.
In Figure 4 this transformation of the positive charge of the ball into a negative charge takes place much more quickly than in Figure 3 by reason of the pointed form given to the rod of the electroscope. In Figure 5 the transformation of the sign of the charge does not take place at all in consequence of the plate-like form of the apparatus, which gives the electricity far too slight a density per unit of surface for it to escape.
The theory of the charge of an electroscope by influence is exactly the same as that of the electrification by means of an electrified sphere placed close to a cylinder, which we discussed in a previous paragraph. The electrified sphere represents the ebonite rod, the cylinder the gold leaves and the ball of the electroscope to which they are fixed.
But if these two instruments are the same, the theory of the origin of the electricity given for the one is valid for the other. We have seen that an electrified sphere placed in the vicinity of an insulated metallic cylinder dissociates the matter of this last and transforms it into electricity. When an electrified rod is brought close to the ball of an electroscope, the metal of this ball is likewise dissociated, and the electricity manifested therein is the product of this dissociation.
4. Mechanism of the Leak of Electrical Charges from Insulating Bodies ~
Electricity is able to diffuse itself in conducting bodies by conduction, convection, and influence. How does its diffusion take place in non-conducting bodies?
Maxwell’s theory is well known. According to him, electricity does not circulate in dielectrics because it would have to overcome an elastic resistance which goes on increasing, and soon opposes its propagation.
It is true that a dielectric may for a very long time retain a part of its charge. I have electrified blocks of paraffin which at the end of 18 months retained a weak residual electrification. But it is evident also that dielectrics very quickly lose a great part of their electricity, because a rod of ebonite excited by friction, of which the potential may exceed 1500 volts, loses the greater part of its charge in a few minutes. It is even astonishing that it should not disappear quicker, if we admit that the quantity of electricity retained by a body is kept on its surface by the pressure of the insulating gas and of the equally insulating ether which surround it. When there is no longer equilibrium between these antagonistic actions, the electricity partly escapes.
My researches prove that this loss of electricity by an insulating body is effected in two ways: (1) by convection -- that is to say, by the emission of particles; and (2) by conduction -- that is to say, by propagation along an electrified body to one of its extremities, as in the case of conductors.
This last mode of propagation, evidently contrary to the reigning theories, can be put in evidence by the following experiment: --
Procure some rods of ebonite or of paraffin about 1 meter long. After having electrified them at one of their extremities by rubbing them for a length of only 2 centimeters, the unelectrified end is placed in contact with a conducting body connected with the ball of an electroscope. No displacement of the gold leaves is noticed at first, but by prolonging the contact for a few minutes the instrument becomes slowly changed. The electricity is therefore propagated along the unelectrified part.
This experiment shows that, in reality, electricity is able to propagate itself in insulators as well as in conductors, but much more slowly in the first-named case than in the second. When we speak of the velocity of electricity, therefore, it must be said that, according to the body employed, it circulates with a rapidity varying from a few centimeters to 300,000 kilometers per second.
Figure 6 ~

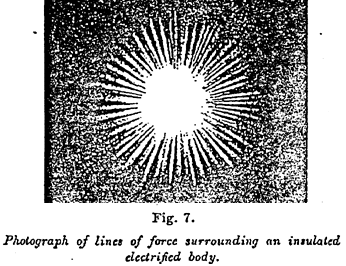
Figure 7 ~
5. Causes of the Differences of Tension Observed in Electricity Proceeding from Chemical Decompositions and from those Produced by Influence or Friction ~
We know that the electricity obtained by the chemical reactions which take place in a battery of any kind is emitted in quantities varying with the size of the battery, but at a tension independent of its dimension. Whether the battery be the size of a thimble or of a house, the tension is always the same and always very weak, since it hardly exceeds two volts per cell.
It is quite different with electricity drawn from matter without the intervention of any chemical reagent -- that is to say, by friction or influence. The quantity of electricity then produced is extremely weak, but its tension extremely great. It may exceed 1500 volts from the friction of a simple rod of ebonite, and may easily attain 50,000 volts from the very smallest static machine used in a laboratory. In order to obtain the same voltage with batteries, it would be necessary -- the elements being added together -- to collect about 25,000 cells.
These differences greatly impressed the old physicists, and was the origin of the division set up between static and dynamic electricity -- so-called -- which weighed down science for over 50 years. Notwithstanding the apparent dissimilarities resulting from differences of tension, the electricity generated by a battery is identical with that produced by a static machine. The battery and the static machine both produce, when their poles are connected by a wire, an electric current surrounded by a magnetic field and able to deviate the needle of a galvanometer.
A battery of which the poles are separated is in every way comparable to a static machine just charged, the poles of which are far apart. Alike between the poles of the machine and those of the battery, a certain difference of potential exists. When they are connected by a wire, the electricity flows one pole to the other, and it is this flow which constitutes the electric current. Since the electricity generated by chemical decomposition as in batteries, and that produced by simple friction in machines, do not differ, why has the electricity of a battery produced by chemical decompositions only a tension of one or two volts, while that obtained by simple friction attains a tension 20 or 30 thousand times as much? The textbooks are silent on this question.
As none of the chemical decompositions known to us intervene in the friction or influence machine, the production of electricity in these latter has probably another origin than that of molecular reactions. It proceeds, as previously t shown, from the dissociation of the atoms. When electricity is drawn from a simple body, whether by influence or by friction, it is simply intra-atomic energy which is liberated. Now, as this latter exists in matter in a state of extreme condensation, it is not astonishing that electricity should go forth from it at high tension. It has, on the contrary, a very low potential when it results not from the dissociation of the atom, but from changes of molecular equilibria. Compared with intra-atomic energies, intra-molecular energies are extremely weak.
I have noted in several experiments, the most important of which has already been mentioned above, the case with which intra-atomic energy transforms itself into electricity at a high potential.
Electrification by friction has always been obtained hitherto by rubbing insulated bodies -- rosin, glass, etc. It is preferable, in order to justify the preceding thesis, to use conducting bodies. By employing simple bodies, such as pure metals, we avoid all foreign factors capable of coming in when such complex substances as glass or rosin are used.
To easily effect the following experiments strips of various metals -- copper, aluminum, etc., in the form of a rectangle about 10 cm square -- must be firmly fixed in ebonite handles. If, holding the ebonite handle in one hand, the strip of metal be slightly rubbed with a catskin held in the other, it will be seen, on bringing the metal close to the electroscope, that it is charged with electricity of a potential from 1000 to 1500 volts. The gold leaves sometimes stand out horizontally, and may even be torn.
The experiment only succeeds when the atmosphere is very dry. If, to dry the metal, it is heated to about 100 degrees or more, it can no longer, after cooling, be electrified by friction otherwise than very slightly. It then regains the property of being electrified at a high potential only after a certain lapse of time, which varies with the metal.
Temperature exercise little influence on certain metals, such as copper; but a great deal on others, such as aluminum. This latter does not regain the property of being strongly electrified for a quarter of an hour after having been heated.
When the metal is in conditions in which it can be easily electrified, it suffices to pass a catskin once lightly over it for it to be electrified to a potential of a few hundred volts. It is therefore quite correct, as I have said elsewhere, that matter need only be touched in order to draw electricity from it. So rapid a transformation by so simple a means always appears extraordinary when one reflect upon it. It is all the more striking that, in electrification by the simple contact of two heterogeneous metals, the potential obtained is only a few volts -- that is to say, so feeble that it cannot be observed with an ordinary electroscope. Volta, who discovered electrification, only succeeded in demonstrating this by means of his condensing electroscope.
If the electrification of metals by friction or by influence did not depend on so many very variable atmospheric conditions, there might be an advantage in replacing the insulating glass or ebonite plates of static machines by metal plates properly insulated.
Very often in the course of this long study we have recorded the ease with which matter can be dissociated notwithstanding its apparent stability, as soon as one brings into action reagents to which it is sensitive. The most powerful forces seem to have no hold upon it; we may pulverize, calcine, and volatilize it without its weight being in any way altered. And yet it suffices to touch it lightly, to let fall on its surface a feeble ray of solar light, for instability to take the place of stability and for its disaggregation to begin. It is from this instability that forces are derived. Electricity, heat, and all the energies of the universe represent unstable forms of matter.
What further strikes us in this study are the colossal energies contained in the smallest particle of matter. These energies play perhaps a preponderant part in biological phenomena. We already obtain a glimpse of this from the importance of the role of substances such as toxins, diastases, and colloidal bodies, containing only imponderable traces of matter, which is, however, doubtless in a form where its energies can be liberated. We evidently find ourselves here in the presence of a new world. The thorough study of which will modify all our present conceptions of the universe.
The Problems of Magnetism, Magnetic Induction, and Lines of Force
1. The Problem of Magnetism ~
The problem which offers itself to us in the case of electrification by influence likewise presents itself as regards the phenomena of magnetism and of magnetic induction.
We know that a permanent magnet can magnetize an indefinite number of iron bars. A limited quantity of magnetism would therefore seem to produce an unlimited quantity.
To say that the magnetism of a magnet only serves to orient the molecules of iron subjected to its action is no explanation at all, for, if it were so, this orientation would necessitate an expenditure of power which would exhaust the magnetism of the magnetizing body.
The interpretation of magnetism being evidently difficult, we still keep to the old hypothesis of the particular currents of Ampere notwithstanding all the objectives aroused by it. That of Lorentz -- on which, however, he does not greatly insist -- is not much more satisfactory. "It may be supposed", he says, "that there exists in a magnet electrons which turn or whirl".
Observation teaches us that there issues from a lodestone lines of force of which the existence and the direction are rendered plain by the classical experiment of the magnetic spectrum. Collectively they constitute what is termed the magnetic field.
Let us keep, then, to this experimental fact, that magnetism is accompanied by the emission of things of unknown structure called lines or tubes of force. Taken together, they form a pencil of which the size depends on certain conditions, such as the section of the magnet. It would suffice, in order to explain the phenomena of the magnetizing of all the bars of iron successively introduced into the field of a magnet, if we admitted that its lines of force are immediately appropriated by the bars of iron they meet, and as immediately replaced by others issuing from the magnet and equal in number.
But we thus only put the difficulty a stage further back. How can a magnet indefinitely replace the line s of force it loses by magnetizing another body?
We can only explain this apparently infinite production by recurring to my theory of the dissociation of matter and of the liberation in immense quantities of the intra-atomic energy it contains. The manifestations of intra-atomic energy are numerous. Heat and electricity are two of them, to which must, no doubt, be added magnetism.
A loadstone can generate an almost unlimited number of lines of force, exactly as a fragment of radium can generate an almost unlimited quantity of heat and of certain radiations. These radiations, again, are capable of being seized upon by neighboring bodies and of giving to them the properties of radium itself. This is the phenomenon which is termed induced radioactivity, and presents great analogies with the magnetism induced by the neighborhood of a magnetized body.
I shall not dwell on the preceding interpretation, because it is impossible to verify its validity by experiment.
Physicists, moreover, have almost renounced the attempt to explain the phenomena relating to magnetism. Poincare has very well shown in the following passage how wretched is the explanation generally admitted: --
"A magnet attracts a current; here, then is mechanical work produced, but whence does it come? There still remains to us, in order not to avow our embarrassment, the resource of a merely verbal explanation, and of saying that the work reaped comes from a diminution of the potential energy of the system. It is indeed in this fashion that the manner in which two permanent magnets act on one another is interpreted -- it cannot be said explained.
"But when we see a current displacing itself under the notion of electromagnetic forces, and we ascertain that we can thus obtain continuous rotations, it seems very improbable that the work obtained can come in this case from a corresponding loss in the potential energy of the system. One must, in fact, suppose that this energy has, so to speak, an infinite, and this supposition is rather repugnant to common sense.
2. The Problem of Induction ~
The same problem is equally present in the case of magnetic induction. How, with a definite quantity of magnetism, can we produce an apparently unlimited quantity of induced electricity?
By cutting the lines of force of a magnet by a metallic wire forming a loop, of which the extremities are connected with the terminals of a galvanometer, we verify during the passage of the wire through the lines of force the appearance of a so-called induction current. It suffices to continuously displace the metallic wire in the magnetic field, so long as the displacements are repeated.
On this fundamental fact of the production of a current by a conductor cutting the lines of force are based our magneto-electric machines, from which dynamos only differ because here an electromagnet takes the place of the fixed magnet. We will only consider here the first-named, which will enable me to make our demonstrations clearer.
In these magneto-electric machines, the single wire referred to above is wound on a drum revolving between the poles of a fixed magnet, so as to cut again and again its lines of force. So long as the coil continues its movement, the magnetism of the magnet will be transformed into electricity. With a finite quantity of magnetism, we therefore produce an unlimited quantity of electricity.
By the current theories, this indefinite generation of electricity at the expense of a limited quantity of magnetism is explained by saying that it is the rotational movement of the coil which is transformed with electricity, or, if you will, that the power expended against the electromotive forces is again met with under the form of electrical energy. Such a metamorphosis would be as marvelous, in reality, as the transmutation of lead into gold by simply shaking it in a bottle. It is very unlikely that kinetic energy should undergo such a transformation, and another interpretation must be sought for the phenomenon.
The explanation propounded, which is the one given above for the phenomena of the same order, rests on no transmutation. Matter being easily dissociated and constituting an immense reservoir of intra-atomic energy, it is enough to admit that the lines of force seized upon by the conducting body which cuts them and causes them to flow in the form of an electric current, are constantly replaced at the expense of the intra-atomic energy. This last being relatively almost inexhaustible, a single magnet can furnish an almost infinite number of lines of force.
The displacement of the conducting body in the magnetic field serves solely to put it in the condition necessary for absorbing the lines of force and giving them the form of an electric current. A determinate quantity of movement and consequently of work is, then, required to generate a certain quantity of electricity; but we are in no way justified in deducing from this the transformation into electricity of the simple movement of a body.
Therefore, when we see at work these gigantic dynamos whence torrents of electric fluid flow, we should not say that they represent movement transformed into electricity. It is simply the intra-atomic energy of dissociated matter which appears under the form of electricity.
Here, again, we perceive the fertility of the principles I have propounded on the dissociation of matter and the magnitude of intra-atomic energy. The immense reservoir of forces contained in matter allows us to explain the majority of phenomena, from the electricity which lights our street to the solar heat whence life is derived.
3. The Problem of the Origin of the Lines of Force ~
Into most of the phenomena we have just examined the lines of force enter. They seem to be the fundamental elements of electricity.
Faraday, starting from the idea, which contravened, however, that of most physicists of his time, that matter cannot act at a distance -- that is to say, where it is not -- deduced therefrom the existence of an intermediary connecting electrified bodies. He was thus led to recognize that these last are surrounded by lines of force throughout the whole space or so-called electric field in which their action is produced.
The illustrious physicist attributed to these lines a very real existence, and by no means considered them as a simple mathematical expression. He regarded them as a kind of elastic springs, repelling each other mutually, and connecting bodies charged with electricities of contrary sign. Their extremities, affixed to these bodies, constituted the electric charge.
Faraday put in evidence the lines of force which surround magnets by the classic experiment o iron filings powdered on a card under which are placed the two poles of a magnet. These filings spread themselves over the card in the direction of the lines of force.
Figure 8 ~
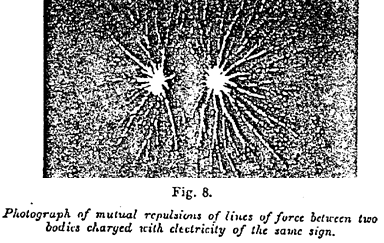
It was supposed by an extension of this theory that Faraday supposed electrified bodies to be surrounded by lines of force analogous to those round the poles of a magnet. At the present day we possess several means of rendering their existence plain (1). They may even be photographed, by using the luminous ions which, during electric discharge, follow their direction. Figure 7 shows the lines of force radiating round an insulated electrified body. Figure 8 shows the repulsion of the lines of force between two bodies bearing electric charges of the same sign.
[(1) That lines of force also appear between conductors carrying an electrostatic charge can be shown by interposing between them a glass vessel filled with oil of turpentine and crystals of quinine sulfate. See Kolbe’s Introduction to the Science of Electricity, 1903, p. 125, et seq.]
Of what are the lines of force composed? Maxwell considered then to be formed of ether cells, whirling round lines serving as axes. Between them would exist the particles which constitute electricity. No experiment allows us to verify this theory. We must confine ourselves to noting the action of lines of force without being able to say anything regarding their structure. They possess properties showing that they are produced in the ether rather than in the matter whence they appear to issue.
On e of the most fundamental of these properties, although it is hardly mentioned in the textbooks, is that of passing through, or behaving as if they passed through, all material substances, whether conductors or insulators. This the x-rays and the particles emitted by radium do to only a slight extent. It is precisely because this property had been almost unperceived by physicists that they were so impressed at the time of the discovery of radiations passing through matter. Bodies can, however, be traversed by lines of force much more easily than by easily than by the x-rays. If the lines of force had been capable of acting on the photographic plate, the x-rays would never have caused the excitement we all remember. The imperfect passage of these last through bodies alone permitted the celebrated photography of the bones of the hand which has been so much talked about.
The experiment of Faraday’s cage, showing that bodies surrounded by a metallic screen connected with the earth cannot receive an electric charge, makes us forget the ease with which metals allow themselves to be transpierced by lines of force. By doing away with the earth-wire, a metal opposes no obstacle to their passage.
Figure 9 ~
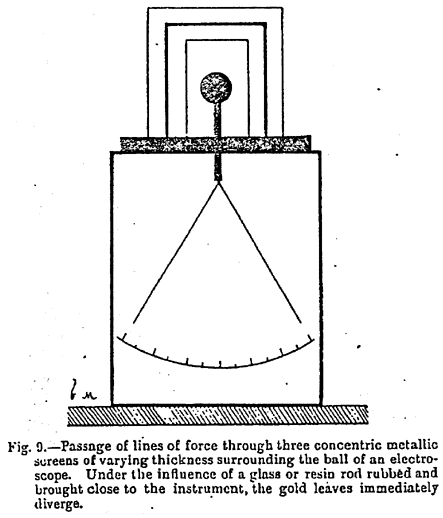
The apparatus shown in Figure 9, which I have constructed in order to render evident this passage of the lines of force through both metals and insulators, proves that an electroscope protected by three concentric cylinders, whether of metal or of ebonite, can be instantaneously charged. Naturally the metallic cylinders are placed on an insulating support.
The transparency of bodies to magnetic lines of force is as complete as to electric lines of force, but is too well known to require demonstration. Everyone knows that a magnet cannot act in a compass through a door or a non-magnetic metallic body.
Nothing, moreover, is easier than to verify this. A compass is placed at a short distance from a magnet. The deviation of the needle is noticed, then a thick plate of non-magnetic metal is interposed between the compass and the magnet. The needle of the compass is not displaced. Therefore the lines of force have passed through the metal without undergoing any trace of absorption, and act on the compass. But if the interposed body were capable of magnetization, it would seize upon the lines of force of the magnet, and would then constitute a magnetic screen. It is just, as before said, this capture of the lines of force which produces magnetization and also magnetic induction when we displace a conductor in a magnetic field.
These lines of force, the properties of which have just been demonstrated, have no analogy with material substances, and yet it is evident they come from matter. The neighborhood of an electrified rod of glass or resin draws them forth from it, and it suffices to withdraw the latter for them to return to it.
Without even bringing an electrified body neat to matter, we can draw from it lines of force. It is enough to put in contact two heterogeneous substances. We then observe that, if the surfaces of the two bodies are drawn apart, they are connected by lines of force which lengthen or shorten as the surfaces are farther apart of nearer. They are real elastic threads, as Faraday recognized, but of what are they formed? If they are composed solely of ether, their property of lengthening indefinitely and of shortening down to molecular dimensions is not easy to interpret.
It is hardly possible at the present day to dispute the very real existence of lines of force. A magnetic field formed by them is a sort of viscous medium, dragging with it the bodies plunged therein when we put it in motion. Masses of metal introduced into a magnetic field follow it in its rotation. Industrial electricity at the present day utilizes these "rotating fields" to put bodies in motion without any material mechanism.
These facts, as yet little capable of explanation, especially tend to put in evidence the close relations of mater with the ether, on which I have so much insisted, and which the science of today makes into two widely distinct domains. When their extreme form -- for instance, a sun ray and a block of metal -- are alone studied, they seem, in fact, very different. But by the examination of the intermediate elements linking up these forms so dissimilar in appearance, we demonstrate that the ether and matter are not two separate worlds, but one and the same world.
The Electric Waves
1. Properties of the Electric Waves ~
In 1888 the physicist Hertz discovered that oscillating electric discharges generate in the ether a series of undulations analogous to those produced by the fall of a body into water. He demonstrated that these waves are propagated, refracted, and polarized like light, and circulate with the same velocity, only differing from light waves by their dimensions. The smallest electric waves are 5 millimeters long, while the largest light waves are 50 microns. The latter are therefore 100 times smaller than the former.
Electric waves produce induction currents in the conductors they meet with. It was by noticing the sparks generated by these currents that Hertz revealed the existence of the waves which today bear his name; and it is on the propagation of these waves in space that wireless telegraphy is based. To reveal their presence at a distance we had only to find a reagent analogous to the ear for sound, or to the photographic plate for light.
The only characteristics common to electric waves and light are their speed of propagation, and their capacity for reflection, refraction, and polarization. But it must be remarked that any periodical disturbance of the ether, whatever be its cause, must necessarily possess similar properties. It is therefore perhaps going too far to deduce from this the identity of the Hertzian and the light waves. This could only be properly admitted if these last also possessed electromagnetic properties, and consequently were able to produce induction currents in conductors. Now, we have never been able to obtain with light waves any electric or magnetic phenomenon, nor to verify their propagation along a wire, as in the case of electric waves.
There would be no interest in dwelling on these differences, as the electromagnetic theory of light -- now generally admitted -- corresponds to that yearning for simplicity and unification so general in physics at the present time. Time alone can stem such currents of opinion.
Abandoning, then, the idea of disputing the value of analogies now universally accepted, we will confine ourselves to the remark in passing that Hertzian are to electricity what radiant heat is to that circulating in matter. A heated body radiates into the ether what are called waves of radiant heat, which raise the temperature of the substances on which they fall. An electrified body suddenly discharged radiates so-called electric waves which charge with electricity the bodies that they meet. There is in this a parallel which, however, in no way leads to identification. The causes which determine the production of waves of radiant heat seem to possess only distant analogies with those of electric waves.
2. Sensitiveness of Matter to the Electric Waves ~
The more we study matter, the more we are struck by its extraordinary sensitiveness. Under its apparent rigidity it possesses a very complicated structure and an intense life. Fragment of metal can be so acted upon at a distance of several hundred kilometers by simple vibrations of the ether, that they become conductors of electric current, the passage of which they at first present. This capacity for impression is another hint of the relations which connect the ether with matter.
The reagent for electric waves employed by Hertz was not very sensitive. It simply consisted of a wire bent into a circle, terminating at its extremities in two balls brought very close together. Moving this receiver about in space, he saw break forth between the balls, on the passage of the waves, small induction sparks produced by their action. Receiving these waves in a large cylindro-parabolic metal mirror, he recognized that it was in its focus that the greatest production of sparks was manifested. Compelling them to pass through a prism of bitumen, he observed that they were deviated. Thus their reflection and their refraction were demonstrated.
Hertz’s receiver was not very sensitive, since it did not reveal the existence of electric waves at more than a few meters from their point of emission. This was fortunate; for if Hertz had made use of the receivers now employed for wireless telegraphy, the phenomena of reflection, refraction, and polarization could only have been discovered with great difficulty. I have often noted, in fact, that sensitive receivers placed in the focus of the Hertz mirror, or by the side of or even behind it, give identical indication, and I shall have occasion to explain why. As has often been the case in the history of science, the rude instrument has rendered more service than a delicate one.
With the receivers of slight sensitiveness employed by Hertz, the apparatus producing the waves had to be very powerful and consequently very bulky. Thanks to the receiver discovered by Branly, the most insignificant sparks suffice, on the contrary, to make an impression (Figure 10).
Figure 10 ~
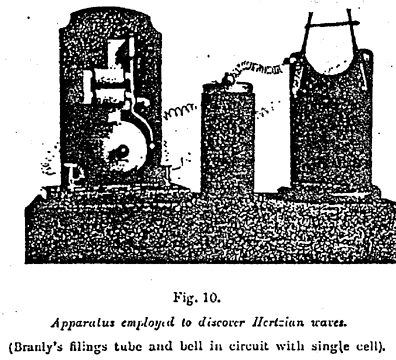
In spite of it marvelous sensitiveness, the receiver called the radio-conductor or filings tube is so simple that any one can easily make it. It consists merely of a small glass tube containing two rods of metal two or three millimeters apart, between which is interposed a small quantity of metal filings. Thus constituted the tube insulates; but on being struck by electric waves sent by a radiator, which may be placed hundreds of kilometers away, it becomes a conductor and allows the current of the battery to pass into the circuit, which also contains an electromagnet designed to operate an ordinary Morse telegraph. A slight blow on the tube, automatically given by another electromagnet thrown into circuit by a relay, serves to bring it back to its original state and allows a fresh signal to be given.
As has just been said, these receivers are so sensitive that the very weakest source of electricity is sufficient to act on them. The difficulty is not to be able to produce electric waves, but rather to manage not to do so when it is desirable to avoid it. Any sudden discharge of an electrified body, however small the spark may be, produces electric waves. They can be obtained by rubbing a rod of resin, by sounding an electric bell, by opening or closing the circuit of a battery, etc. I have caused a receiver to act at a distance of 50 centimeters away by the spark from a simple ebonite rod rubbed with a catskin and afterwards touched by a metallic object of fairly large capacity such as a key or coin.
The discovery by M. Branly of the variation in conductivity of metal filings under the influence of the Hertzian rays, certainly constitutes one of the most remarkable discoveries in modern physics, especially because it opens up unforeseen horizons on phenomena still very mysterious, and on which the sagacity of physicists will no doubt have to exercise itself for a long time.
We have here a very general fact which is in no way limited to the case of metal filings. This is that fragments of metal in contact with one another and presenting considerable resistance -- 30,00 ohms, for example -- to the passage of electricity, become conductors under the influence of very weak electric waves, and lose this conductivity by a simple shock. These variations indicate a great variability in the aggregation of atoms under the influence of infinitely weak but appropriate forces.
Most discontinuous conductors can exhibit these variations. Now we hardly handle any other than discontinuous conductors. A continuous conductor, such as a metal wire, becomes discontinuous immediately we connect it to the terminals of a battery. And we have just seen that in a discontinuous conductor the resistance is not at all -- at least for a number of metals -- a constant magnitude depending on the section and the length of the wire according to Ohm’s law, it follows that the conductivity of certain metallic bodies may vary in immense proportions according to the pressure of the wires by the binding screws and the circumstances in which they are used. Even with a constant pressure this resistance may still very considerably, since it suffices to cause an electric spark in their neighborhood in order to modify the resistance of the contact. Now the simple fact of opening o closing a current produces such sparks. A filings-tube with a variable resistance is only an exaggeration of these effects.
It is easy to illustrate what has just been said by a curious experiment due to M. Branly. A column of metal of 30 or 40 cm in height is formed by placing one upon the other a series of polished discs of bismuth, aluminum, etc., of the dimensions of a 5-franc piece. The column may even be made merely of polished steel balls placed in a test tube. The resistance of this column to the passage of an electric current is still considerable. If we make a small electric spark near it, the resistance becomes almost nil. To cause it to reappear, it suffices to give a tap to the upper part of the column.
These variations are still more exaggerated if the column, instead of being formed of discs of the same metal, is composed of discs of different metals. With a column of alternate discs of lead and aluminum, a few taps will suffice to raise the resistance to about 30,000 ohms, and very slight electric waves sent from a distance reduce this enormous resistance to 3 ohms.
This variability of resistance is not observed in all metals in contact, for copper is a notable exception, and it is fortunate it is so. The law of Ohm I = E/R naturally implies the knowledge of R. Now, if the physicists who established it had used wires of different metals other than copper, it could not have been discovered. They would have noted, in fact, by using any measuring apparatus whatever -- the Wheatstone Bridge, for instance -- that the resistance constantly varies in large proportions for a given length of wire, and that the variations of pressure in the binding-screws did not explain this phenomenon. Even had they noticed the influence of a spark emitted close by -- and the opening or the closure of the battery current employed always produces one -- they could not have deduced any measurement from it, since the effects of these sparks vary greatly from experiment to experiment. The final conclusion would no doubt have been that a wire of constant dimensions may in apparently identical circumstances allow very different quantities of electricity to pass, which is directly contrary to Ohm’s law. This law, which is the basis of all our practical knowledge of electricity, would no doubt have been discovered, but much later and by very roundabout ways.
The property peculiar to a small number of metals of remaining uninfluenced by electric waves seems able to communicate itself to neighboring bodies, as if these metals possessed a kind of metallic atmosphere. Let us take a column of copper discs, which metal has an invariable conductivity. The resistance of this column will be a constant magnitude depending on the height and diameter of the discs, and will consequently obey Ohm’s law. Take a second column of aluminum discs, a metal of variable conductivity, and their resistance will vary in considerable proportions according to the conditions, such as shock, conveyance of electric waves, etc, enumerated above. Let us now mix the discs of the two columns in such a way that a disc of copper follows one of aluminum. The copper communicates its properties to the aluminum, and the column behaves as if it were composed solely of copper. Its resistance has become invariable; it now obeys Ohm’s law.
3. The Propagation of Electric Waves at a Distance and Their Future Utilization ~
We have seen that Hertzian waves propagate themselves in space to a distance of hundreds of kilometers, and produce on the metallic bodies they meet electrical induction currents capable of manifesting themselves in the form of sparks.
This production of electricity at a distance is very slight, because the electric waves disperse their energy to all directions of the horizon (1). But it would be quite otherwise if we could concentrate them on one point by means of mirrors or reflectors, as we do with light. Such reflectors (cylindro-parabolic mirrors) were indeed used by Hertz in his experiments, since it was owing to them that he demonstrated the reflection of the electric waves; but unfortunately, by reason of the size of these waves, and the consequent phenomena of diffraction, the greater part of the energy is lost. To concentrate it, mirrors of gigantic dimensions would be necessary.
[(1) But not equally. Dr Marconi’s experiments have shown that the Hertzian waves emitted from the antenna of a Marconi apparatus, for instance, are projected farther in one direction than another, the curve of their activity taking the form of a pear, of which the antenna represents the stalk.]
However, I do not believe the solution to the problem is impossible, and if I have not carried out the plan of researches on this subject which I intended to effect, it is solely on account of the expense they would have necessitated. I intended to place side by side, and in contact with each other, a large number of cylindro-parabolic mirrors. These need not have been very high, for we can easily induce the waves to form in a plane of slight thickness. I should then have tried to produce extremely intense electric fields, such as we can obtain with divers instruments -- the Oudin resonator notably. When this apparatus works with a coil having a 60 cm spark, all the metallic objects in a room 10 meters long are charged by induction to such a high degree that they spontaneously emit thousands of sparks. They once produced in my presence, at a distance of several meters, short-circuits in a switch-board containing voltmeters and ammeters, etc, connected by wires with a double coating of insulating material. In the result, these short circuits caused the wires to melt, and the experiment had to be immediately stopped for fear of fire.
The problem of sending a pencil of parallel Hertzian waves to a distance possesses more than a theoretical interest. It is allowable to say that its solution would change the course of our civilization by rendering war impossible. The first physicists who realizes this discovery will be able to avail himself of the presence of an enemy’s ironclads gathered together in a harbor to blow them up in a few minutes, from a distance of several kilometers, simply by directing on them a sheaf of electric radiations. On reaching the metal wires with which these vessels are nowadays honeycombed, this will excite an atmosphere of sparks which will at once explode the shells and torpedoes stored in their holds.
With the same reflector, giving a pencil of parallel radiations, it would not be much more difficult to cause the explosion of the stores of powder and shells contained in a fortress, or in the artillery parks of an army corps, and finally the metal cartridges of the soldiers. Science, which at first rendered wars so deadly, would then at length have rendered them impossible, and the relation between nations would have to be established on new bases.
It must not be supposed that by any means whatever that a ship or fortress could be protected from the action of the Hertzian waves. No doubt our experiments have proved, as we shall see in a later chapter, that a sheet of metal 1/100th of a millimeter thick completely stops the electric waves, but the same experiments have also proved that this theoretical protection is entirely illusory. The slightest crack between the joints of an enclosure permits the Hertzian waves to pass without difficulty
There would be no interest in dwelling further on this subject. I have more than once in my researches come across problems the solutions of which would modify the march of civilization more profoundly than all the changes of constitutions and reforms. It is only in the progress of science that great social transformations can be looked for.
Transparency of Matter to Electric Waves
1. History of the Ideas Relating to the Transparency of Bodies to Hertzian Waves ~
The ideas current on the transparency of bodies to the Hertzian waves were very erroneous when I published my researches in 1899. Experiment seemed to indicate that all metals of slight thickness and insulating bodies of any thickness could be transpierced by the,, but both these views were inexact. At the beginning of his researches, Hertz recognized that metals reflected the electric waves and consequently presented a certain opacity; but was this opacity total or partial? This was the point left unascertained.
In the minds of most authors transparency appeared probable in the case of slight thicknesses. "M. Joubert discovered that a wall of zinc 2-5 mm thick, 4 meters high, and 8 meters long, weakened the sparks without completely destroying them, and that they could still be observed on the other side of this wall" (Quoted by Poincare, Electricity et Optique. Poincare points out that it might perhaps be explained by diffraction, which is indeed the correct explanation, as we shall see alter.)
Sir Oliver Lodge, who has made numerous experiments on Hertzian waves, likewise thought he had noticed the transparency of metals, but supposes that at a 'reasonable' thickness they must be opaque (1). We are ignorant as to the value to be attached to what the author understands by "reasonable thickness".
[(1) Lodge, The Work of Hertz and Some of his Successors; 1891)
Prof. Bose, to whom we owe a very complete memoir on the Hertzian waves, and who has invented some very ingenious apparatus to measure their length, has likewise arrived, by precise and apparently demonstrative experiments, at the conclusion that metals are transparent to Hertzian waves. I here give the translation of the passage of the memoir in which he narrates how he was led to this conclusion. After explaining all the precautions taken to enclose his instruments in a perfectly closed metal box, the learned physicist adds: --
"Notwithstanding all these precaution, I was baffled for more than 6 months by an unknown cause of error which I could not discover for a long time. It was only recently, when almost convinced of the uselessness of continuing my researches, that I discovered that I was mistaken in supposing that the tinplate sides of the case were [perfectly opaque to electric radiations. The metal box containing the radiator seems to transmit a few radiations though its walls, and if the receiver is sensitive it indicates this slight transmission. I than had a second metal envelope made, and found this precaution to be efficacious so long as the receiver was not placed to close to the radiator. Notwithstanding these two metallic envelopes, the receiver is still affected if placed immediately above the radiator" (1).
[(1) Chandahar Bose: "On the Determination of the Wavelength of Electric Radiations by a Diffraction Grating" (Proc. Royal Soc., Oct. 16, 1896]
More recently still, M. Henri Veillon made some analogous experiments in the Physical Laboratory of the University of Bale. They had as object the discovery of "the part played by conducting bodies placed between the receiver and the transmitter from which come the oscillations". After acknowledging the difficulty of the subject the author declares that he only ascribes to his experiments "the character of a simple contribution to a study offering great difficulties". The most important of his experiments is the following: -- The receiver was placed in a zinc case 1 mm thick closed by a lid sliding in a groove, with the radiator outside. Under these conditions the experiment demonstrated to him that the action of the waves transmitted by the radiator "passed through the metal envelope, but only when the sparks did not pass at too great a distance (about 1.5 meters)" (2). This conclusion, we see, is the same as that of Prof. Bose.
[(2) Archives Sciences Physiques et Naturelles de Geneve, May 1898 ]
It results clearly from the above extracts that the most exact researches seemed to prove the transparency of metals to Hertzian waves. They appeared very conclusive. They were not, as we shall presently see.
As regards the transparency of conducting bodies it was considered as absolute. At the outset of his researches, Hertz noticed the transparency of dielectrics -- sulfur, wood, glass, etc. It is even by their means that he succeeded in demonstrating the phenomenon of the refraction of electric waves by large prisms of bitumen.
Since then, dielectrics have always been considered very transparent to electric waves, and it has even been inquired whether they might not give a trace of absorption. Righi, in his researches, arrived at this conclusion: "That it can be considered demonstrated that the diminution of intensity to which the radiations passing through the plates of certain dielectrics are subject, are really due to absorption".
Wireless telegraphy experiments seemed to confirm this hypothesis absolutely. The majority of observers have noted, in fact, that walls, and even hills, were traversed by electric waves, and this observation is still reproduced in several works which have become classic.
I therefore found myself in presence of two problems: --
(1) Can metals of slight thickness be traversed by Hertzian waves? (2) Are non-conducting bodies of any thickness transparency to them?
These problems had for me a very great theoretical importance. Their solution was very delicate, and as they necessitated a laboratory of larger dimensions than I possessed, I asked Prof Branly to be kind enough to participate in these researches. We together published the results obtained in the Comtes Rendu de l’Acadamie des Sciences (April 1, 1899, p. 879).
The difficulties of all kinds we had to overcome before reaching a definite result thoroughly explain the erroneous conclusions to which very skillful physicists had been previously led.
2. Opaqueness of Metals to the Electric Waves ~
In order to ascertain whether metals in thin or thick plates allowed the electric waves to pass through them, M. Branly and I had some cubical boxes about 50 cm square constructed of different metals, and between 0.02 mm and 2 mm thick, in which the receiving apparatus was placed. The apertures of these cases were closed by a most closely-fitting door. The radiator producing the waves was placed a few inches away from the apparatus, and the receiver was of course put inside the box. It revealed the passage of the electric waves through the walls by causing a bell to ring (Figures 11 and 12)
Figure 11 ~
Figure 12 ~
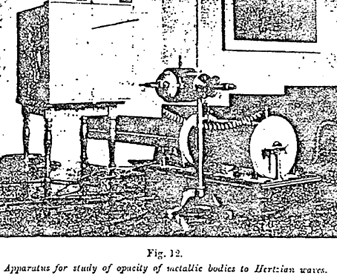
The first experiments seems to confirm absolutely the researches above quoted. Like Mr Bose and other experimenters, we noticed that the electric waves seemed to pas through the metal. The moment the radiator began to act, the bell inside the metal box could be heard.
Although these experiments agreed with those of the authors quoted, we would not be satisfied with them. We had the metal doors readjusted by fitting to each of them half-a-dozen screws in such a way that we could close them hermetically. When these precautions were taken, we noticed that as soon as the screws were tightened, the bell remained silent. The radiations therefore did not pass through the metal; they simply passed through the very narrow gaps left by the metal doors. The transparency observed by former observers was due solely to the imperfect closing of the metal boxes in which they enclosed their apparatus. The precautions taken by photographers to protect their laboratories from the entry of light would be quite insufficient to protect them from the entry of the electric waves.
These experiments were repeated many hundred of times with the same result -- tightening of the screws, silence of the bell; loosening of the screws, ringing of the bell.
We then tried with several metals, the effect produced by the thickness of the envelope, and it proved absolutely nil. A case of thin wood, lined with tin and only 1/100 mm thick, proved to be as adequate as boxes of metal of 2 mm.
The protection exercised by a metal envelope is quite remarkable. In the metal door which closes the case let us solder, perpendicularly to its center, a brass rod projecting 30 cm inside the box, and protruding as much outside (Figures 11 and 12). Let us connect the circuit of the filings-tube to the inside portion of this rod, and place its outside part in contact with the radiator. It would seem that under these conditions the receiver ought to act. Now, it does nothing of the kind. Though the electric waves are propagates, as we know, along wires, that part of the rod which is inside the metal box entirely ceases to conduct. As the walls of the case form a screen, the electric waves take the shortest road, which is, evidently, the outer surface of the metal cage. By touching the latter with the finger during the experiment we can draw from it numerous sparks.
The above experiments, and notably that which consists on causing or arresting the passage of the electric waves by tightening or loosening the screws closing the door of the metal case, prove that the tiniest slits allow the passage of the electric waves. Being thus led to examine the influence of these slits, we noted that if we replaced a slit intentionally made in the metal box by a series of holes the total area of which greatly exceeded that of the slit, the passage of the electric waves took place through these holes much less easily than through the slit. The radiator only needed to be placed a few meters farther off, and the bell remained silent. Thus one hundred round holes of 1 cm diameter do not give passage to the electric waves so long as the radiator is more than 50 cm distant, while with a crack 1 mm wide by 20 cm long, the receiver in the box was affected at all the distances at our disposal, and even when the radiator was placed in an adjacent room. With a slit made by simply passing the edge of a razor across the metal, the outer box was still transpierced by the waves, but at one-sixth of the distance employed with the slit of 1 mm.
If the slit is perpendicular to the axis of the four balls of the radiator, the box is transpierced from a distance 6 to 8 times greater than when the slit is parallel to that axis.
These experiments led to the presumption that a wire gauze of fine mesh would act exactly like a solid sheet, and this was easily verified by observation. An envelope of wire gauze, with meshes 1 mm square, is opaque, save when the radiator is only a few centimeters away.
From what precedes, it results that metal envelopes act with regard to the electric waves much as Faraday’s cage does with regard to electrostatic induction. It will be remarked. However, in the case of electric waves, that the slits exercise an influence which is not produced in the case of static electricity. There must be likewise observed in electric waves the facility with which they pass through very narrow slits while square openings large enough for the insertion of a finger will not allow them to pass. Electric waves appear to behave as if they were rigid and similar to a metal disc which passes without difficulty through a sufficiently large slit, but is stopped by an orifice smaller than its diameter.
We often indeed had occasion, in the course of our experiments, so observe the facility with which the electric waves travel round obstacles. This is evidently a diffraction phenomenon depending on the size of the waves produced by the radiator employed. Whether, in the preceding experiments, the radiator be placed before, behind, or at the side of the face of the box containing the receiver, with an imperfectly fitting metal door the bell will act equally well. It is precisely for this reason that, with apparatus as sensitive as ours, it would have been very difficult, as has been said, for Hertz to effect his researches on the refraction, reflection, and polarization of the waves of which he discovered the existence. If Lodge was able to repeat them with filings-tubes it is because, from the very nature of their construction, they possessed no greater sensitiveness than the receivers of Hertz.
It is the passing round obstacles through the effects of diffraction which has above all contributed to deceive observers as to the transparency of voluminous bodies to electric waves. Whether it be a liquid, an electric, or an acoustic wave which is in question, an obstacle is all the more effectively passed round the greater the wavelength relatively to the dimensions of the obstacle. The sound of a flute played behind a house is less perceptible than a trombone, precisely because the sound waves produced by the trombone are longer than those produced by the flute, and consequently pass more easily round the house. Electric waves, being very large, easily pass round large obstacles; while light rays, being very small, can only pass round obstacles of the dimensions of a hair. It is for this reason that the phenomenon of diffraction, so easy of observation, has been so difficult to establish in the case of light. It is solely because he could not observe it, that Newton contested the wave theory of undulations. If light, said he, were constituted of waves, bodies would have no shadows, because the waves would turn round their edges as sound turns a corner and liquid waves a rock.
From what has been said, we may conclude that rigidly closed metallic screens, however slight the thickness of the metal, offer an absolute obstacle to the passage of electric waves.
It will not be without its use to point out that in practice the protection afforded by metallic envelopes will always be illusory, because it is extremely difficult to make hermetically closed envelopes.
Our researches prove that metals are opaque to electric waves, but their transparency, which seemed, moreover, demonstrated by all the early experiments, offered nothing improbable. This transparency, no doubt, did not agree with Maxwells’s equations, but as one can never get anything out of an equation which has not been previously put into it, it would have sufficed to put in something else to have drawn out quite different conclusions. Metals are very transparent, as we saw in another chapter, to electric and magnetic lines of force, and nothing proved absolutely that they were not so to electric waves.
It was, therefore, experiment alone that allowed the question to be decided.
3. Transparency of Non-Conducting Bodies to Electric Waves ~
The transparency of non-conducting bodies to the Hertzian waves, which has been known since the first researches of Hertz, is confirmed by all the experiments of wireless telegraphy. But is this transparency complete? Do the Hertzian waves really pass through hills and houses, as we have been taught?
One may ask -- (1) If the transparency of non-metallic bodies does note vary with the bodies and also with their thickness? (2) If the transparency of very voluminous bodies like hills is not merely an appearance resulting from the fact that the electric waves, resembling in this sound waves, pass round obstacles.
To resolve these questions definitely, the following experiments were effected in conjunction with M. Branly: --
We constructed a box of Portland cement with sides 10 cm thick, of which the side left open could be closed by a metal door carefully adjusted and capable of being hermetically closed by screws. The instruments designed to reveal the passage of the waves -- that is to say, a needle galvanometer, a battery, an electric bell, and the filings-tube which becomes a conductor as soon as it is struck by the electric waves -- were placed in this box before it was closed.
After hermetically closing the case, and placing close to it a radiator with balls operated by a 15 cm spark coil, the apparatus was put in operation. From the first spark we knew by the ringing of the bell that the sides of the box were perfectly transpierced.
The experiment was repeated by moving the radiator gradually farther and farther off, and we verified, by the silence of the bell, that from more than 7 meters away no electric wave passed through the case. It then sufficed to loosen the screws of the door to hear the bell ring, which showed clearly that the walls of the case constituted the sole obstacle to the passage of the electric waves. After drying for several days, the case became a little more transparent, but still the radiator ceased to act at a distance greater than 12 meters.
These first experiments proved that if an enclosure surrounded by a wall of mortar 12 cm thick allows the electric waves to pass, it still exercises a notable absorption, and becomes quite opaque at a little distance.
To confirm this apparent influence of the thickness of the surrounding wall, we caused to be constructed a second box of cement, similar to the first, and only differing in that its walls were 30 cm thick. Twelve house after it was finished, and while it was yet quite damp, it showed itself absolutely opaque to the Hertzian waves, even when we placed the radiator a few centimeters from its sides. As it dried, it allowed a few waves to pass through, but only when the radiator was not farther off than about 1 meter. Beyond that distance, it remained opaque.
These experiments confirmed the first, and showed that the absorption increased, as might have been supposed, with the thickness. They seemed to indicate also that water possessed noticeably absorbent properties.
To verify this fact, we had a wooden box constructed with walls 30 cm thick and filled with dry sand. In the middle was left a cavity closed by a metal door, arranged to receive the apparatus revealing the waves.
Everything being thus ready, the radiator was put in operation, and we found that the sand behaved like an absolutely transparent body, exercising no absorption, perceptibly at least, at the distance of 40 meters, which was all that was at our disposal.
We then poured into the case as much water as the sand would absorb, and, on repeating the experiment, a considerable diminution in the transparency was observed.
The ease with which the dry sand allowed itself to be traversed led us to suppose that coarse-grained bodies such as stone might be more easily traversed than cement.
To verify this we had cut a block of building stone of 1 meter cube, in the interior of which was left a little cavity to contain the receiver of the waves. This cavity was closed by a metal door carefully adjusted.
The thickness of stone which the electric waves had to cross to reach the receiver was 10 cm. This thickness was, however, traversed without difficulty, even when we placed the radiator at the extreme limit of the garden where we were working -- that is to say, about 40 meters from the receiver. Stone was therefore much more transparent than cement.
The stone was then wetted for several days, and at once its transparency diminished. It was no longer traversed except when the radiator was placed within 25 meters distance.
In the preceding experiments, the dry sand and the dry stone seemed quite transparent, but this was only in appearance, and resulted from the fact that we had not space enough at our disposal to put the radiator far enough off to verify the absorption. To convince oneself of this, it is sufficient to reduce the intensity of the waves emitted by the radiator by using a smaller induction coil -- which comes to exactly the same thing as if the distance were increased with a higher source of radiation. It is then easily verified that the sand and the stone exercise an important power of absorption, and are by no means completely transparent. With a radiator operated by a coil giving sparks of 2 cm only, the block of dry sand of 30 cm thick is no longer traversed at a distance greater than 16 meters, not the block of dry stone beyond 13 meters.
The above figures have, evidently, nothing absolute about them. They simply prove that the transparency of bodies to electric waves is by no means complete, as has been supposed. It is evident that according to the intensity of the source, differences of absorption will be noticed. Waves of 100 meters in length pass through bodies better than waves of 20 cm, but it is equally certain that there must always be some absorption, and that, consequently, mountains and houses are not traversed as was formerly supposed.
I will sum up all that concerns the transparency of dielectrics to the Hertzian waves in the following propositions: --
(1) The transparency of non-metallic bodies to Hertzian waves depends on their nature, and varies considerably from one body to another; (2) this transparency is always much greater than that of these same bodies to light; (3) absorption increases in proportion as the thickness of the body in question increases; (4) humidity greatly increases the absorption; (5) when the electric waves meet great obstacles, such as hills, these obstacles are passed round and not transpierced.
Though the practical deductions which may be drawn from our researches do not here concern us, it may not be without interest to point out that the preceeding observations may find applications in the establishing of wireless telegraphy stations. The electric waves being in part absorbed and consequently weakened by the small obstacles they meet, and obliged to pass round the large obstacles, which likewise weakens them, it will always be useful to place the emitting and receiving stations on elevated points. The crossing of arms of the sea and the communication of continents with islands, constitute for the above reasons the best conditions of transmission.
The Different Forms of Electricity and Their Origin
1. Does the Electricity Derived from Matter Exist in Matter?
According to a theory which in the last few years has become a dogma for many physicists, matter is composed entirely of electric particles called electrons, and its most fundamental property, inertia, is of electromagnetic origin.
My work on The Evolution of Matter has already shown how little foundation there is for this hypothesis. But in order not to complicate an already difficult statement of novel things, I have kept to the almost classic terms derived from this theory. It can, moreover, in no way interfere with my statement, since things happen in appearance as if electricity existed completely formed in matter, instead of being, as I maintain, a secondary transformation of intra-atomic energy.
The theory of electrons is now popular. The proof of this will be found in the following passage of a speech made by Mr Balfour, then Prime Minister of England, at one of the recent meetings of the British Association for the Advancement of Science. The speaker defends in it the new ideas with all the ardor of a neophyte: --
"Learned men", he said, "have come to look on brute matter as a simple appearance, of which electricity is, physically, the real basis, and to think that the elementary atom of the chemist is nothing more than a systematic grouping of electric monads or electrons -- which are not electrified matter, but electricity itself".
This theory, which everywhere confounds cause and effect, is not derived from mathematical arguments alone. It is also based on the interpretation of certain observations, such as the composition of the emissions of radioactive bodies, the phenomenon of Zeeman, etc. But the fact which -- unconsciously at least -- has perhaps most influenced the idea of the learned is certainly the facility with which electricity is drawn from matter. It may be said, without too much exaggeration, that it is impossible to touch matter without causing electricity to come forth from it. It might also be said, and this time without any exaggeration at all, that a body cannot be touched without heat coming forth from it. No one, however, dreams of asserting that matter is composed of calorific particles.
If the fluid called electricity existed complete in matter, it would be in an utterly inexplicable state of condensation. We know, in fact, that it is easy to extract from a small particle of matter a quantity of electricity out of all proportion to that which we can retain upon bodies of great volume. A small part of the 96,000 coulombs drawn from the decomposition of 9 grams of water would charge with electricity to a potential of 7000 volts a globe as large as the earth.
It must be admitted that if electricity exists in matter, it is found there in a form which we cannot conceive. It is impossible to imagine a kind of condensation by the drawing together of the electrons supposed to compose the electric fluid, for they would exercise with regard to one another such immense repulsions that matter could not exist for a single instant. In my last book we saw that, if a charge of 1 coulomb would be concentrated on a small sphere and brought within 1 cm of another sphere bearing a similar charge, the work produced by its displacement at a velocity of 10 cm per second would be equal to 82,000 million kilogram-meters, or over one billion horsepower in the same space of time.
But if electricity does not exist in matter, how can it be explained that it is so easy to draw the first-named from the second? When matter was considered as an inert thing only restoring the energy with which it had been at first supplied, no answer could be given to this question. Since I have shown that, contrary to the old belief, matter constitutes a colossal reservoir of a special energy -- intra-atomic energy -- it has become easy to explain how it is possible, at the expense of this energy, to obtain heat, electricity, or any other force. Without invoking any unknown phenomenon, I have shown how a tiny atom contains an immense quantity of energy. By calculations needless to reproduce, I have shown that a sphere the size of a pin’s head, revolving on its own axis with the speed of projection of the cathode particles, represents an amount of kinetic energy equal to that produced in an hour by 1500 steam engines of 500 hp each.
The rotary movements attributed to the elements of matter alone explain how the particles of radioactive bodies are projected into space with a velocity of the same order as that of light.
These velocities of rotation are necessary not only to explain the projections just mentioned, but also the equilibrium of the elements of which the atoms are formed. In the same way as the top falls to the ground the moment it ceases to turn, the elements of matter only keep themselves in equilibrium by their movements. If these last were stopped for a single instant, all bodies would be reduced to an invisible dust of ether, and would no longer be anything.
This is why it has been possible to compare the atom to a small solar system composed of particles gravitating round one or several centers at some immense velocity. So soon as, from some cause or another, the centrifugal force resulting from the rotation of these elements exceeds the force of attraction which keeps them in their orbits, the particles of the periphery escape into space by following the tangent of the curve they pursue, like a stone hurled from a sling.
In my last book, we considered electricity as an intermediary substance between matter and the ether, resulting from certain disturbances of equilibrium of the ether following upon the partial disaggregation of the atoms. According to the nature of these disturbances, light, heat, electricity, etc., result; but the elements which produce these effects have in themselves nothing electrical or calorific.
If, therefore, matter can, by dissociating itself, produce various energies -- light, heat, electricity, etc. -- this does not in any way say that it is composed of light, heat or electricity. The conception of electrons, a near relative to the old phlogiston, is, as has been well shown by Prof de Heen, one of the most unfortunate metaphysical ideas recently formulated.
2. The Various Forms of Electricity ~
The notion of a disturbance of equilibrium as the origin of any kind of force, is fundamental, and must always be present in the mind in order to understand the various forms of energy. Particles at rest are no more electricity than the ether at rest is light. When the equilibrium of this ether is disturbed, it experiences certain vibrations which we call light. As soon as these vibrations cease, the ether regains its equilibrium, and the light disappears. It is the same with electricity. As soon as the particles constituting the fluid termed electric are no longer in equilibrium, then, and only then, appear the phenomena called electricity. They disappear when these particles regain their equilibrium. One ought no more to speak of neutral electricity than of neutral movement, neutral heat, or neutral light.
All forms of energy being produced by disturbances of equilibrium, it results from this that by varying these disturbances we generate different forces. To find new means of modifying the equilibria of matter and of the ether, is to discover new energies. So long as the equilibrium of the ether could only be modified in one single fashion, we observed only light. When we learned how to create new forms of equilibrium, we obtained the action of induction, Hertzian waves, x-rays, etc.
It is entirely because the means of modifying the equilibrium of the ether and of matter are now becoming multiplied that we witness the discovery of new manifestations of energy. Science classifies them with much difficulty, not being able to connect them with things already known. The confusion which results from this will be easily made plain by the examination of the various phenomena which are still classed at present under the name of electricity.
The following enumeration comprises forms of energy, very dissimilar, but possessing the common characteristic of being able, directly or indirectly, to produce what is called an electric charge. This is the only reason which permits things so different to be classed under the head of electricity. If we adopted in a general way this very summary process of classification, we should have to call heat all the causes which always produce it -- for example, friction and movement.
(1) The Electric Fluid ~ We suppose it to be constituted by the union of the electric particles to which reference will be made later on. After having admitted the existence of two fluids -- the one positive, the other negative -- the tendency now is to recognize only one, formed of negative elements. The positive fluid would thus be composed of material particles of some of their negative corpuscles.
(2) Static Electricity ~ Formed by the accumulation of the electric fluid on a body. Is characterized by the power of producing attractions and repulsions but exercises no action on a magnet.
(3) Dynamic Electricity ~ When a body charged with static electricity is connected by a conducting wire to an unelectrified body, it flows into this wire with a velocity which may reach that of light, and produces an electric current by the sole fact that the electric fluid is in motion; the wire which conducts it is itself surrounded by a field called magnetic, because it enjoys all the properties of a magnet.
(4) Magnetism ~ Particular forms of electricity characterized by equilibria of unknown nature. Magnetism can in certain conditions generate an electric current [unipolar dynamo] in the same way that the electric current can generate magnetism.
(5) The Electric Atoms or Electrons ~ Particles called electric, of definite magnitude, supposed to be constituted by vortices of the ether. May exist without material support in Crooke’s tubes, and in the emissions of radioactive bodies. Can pass through metal plates when their velocity is sufficient.
(6) Cathode Rays ~ Formed by the projection of electric particles in a tube through which a current passes after a vacuum has been created in it.
(7) X-Rays ~ Constituted by disturbances in the ether of a form still unknown, and taking birth when the cathode rays strike an obstacle. Pass through thick plates of metal. Possess no magnetic or electric property.
(8) Negative Ions ~ Supposed to be formed of electric particles surrounded by attraction with material elements.
(9) Positive Ions ~ Must be constituted by material atoms having lost their negative particles. Have never been isolated (1).
[(1) It seems to me that the author here loses sight of the significance of his own experiment described in The Evolution of Matter (p. 385 and Figure 62). The "elements coming from the dematerialization of matter" which he there shows as passing through sheets of dielectric substances can be nothing but "positive ions", that is to say, sub-atomic particles of matter associated with a positive charge. It is difficult to see any distinction between these and the "Ionic Fluid" next mentioned. -- Ed.]
(10) The Ionic Fluid ~ Formed of positive or negative ions mixed with gaseous particle. Can circulate through a metal worm before the elements of which it is formed recombine.
(11) Neutral Electricity ~ Form of electricity totally unknown of which no reagent can reveal the presence, and supposed to be constituted by the union of the positive and negative fluid. Must exist in bodies in a state of extreme condensation, since there can be taken from one gram of water a quantity of electricity much exceeding that which it would be possible to keep on a globe the size of the earth.
(13) Electric Waves ~ Disturbances of the ether which accompany electric discharges and are propagated by vibrations in the ether with a velocity which can attain that of light. They are to ordinary electricity what radiant heat is to the caloric phenomena of which matter may be the seat. By induction, the electric waves may generate at a distance on the bodies they strike currents of electricity capable of manifesting themselves in the form of sparks.
Thus, then, by the sole fact of producing in the ether or in matter dissimilar disturbances of equilibrium, we create very different forces. It is only our need of simplification which leads us to put them together. We might evidently even further simplify them by saying that these energies only represent transformations of movement, but a definition of this kind would apply to all phenomena, including those of life. Such generalizations only translate our ignorance into other words.
The Problems of Heat and Light
Chapter I
The Problems of Heat
1. Old and New Ideas on the Causes of Heat ~
Hardly any scientific subject has called forth so many researches as heat. Thanks to them, thermodynamics and the energetic theory, which are derived from it, have become precise and fertile sciences.
But if we simply ask ourselves what these researches have revealed as to the causes of heat, we are bound to acknowledge that we are hardly more advanced than we were a century ago. We cannot find much to add to the following lines written by the illustrious Humpry Davy, a hundred years ago:
"Since all matter may be made to fill a smaller volume by cooling, it is evident that the particles of matter must have space between them; and since every body can communicate the power of expansion to a body of a lower temperature, that is, can give an expansive motion to its particles, it is a probable inference that its own particles are possessed of motion; but as there is no change in the position of its parts as long as its temperature is uniform, the motion, if its exists, must be a vibratory or undulating motion, or a motion of the particles round their axes, or a motion of particles round each other.
"It seems possible to account for all the phenomena of heat, if it be supposed that in solids the particles are in a constant state of vibratory motion, the particles of the hottest bodies moving with the greatest velocity, and through the greatest space; that in fluids and elastic fluids, besides the vibratory motion, which must be conceived greatest in the last, the particles have a motion round their won axes, with different velocities, the particles of elastic fluids moving with the greatest quickness; and that in ethereal substances the particles move round their own axes, and separate from each other, penetrating in right lines through space. Temperature may be conceived to depend upon the velocities of the vibrations; increase of capacity on the motion being performed in greater space; and the diminution of temperature during the conversion of solids into fluids or gases, may be explained on the idea of the revolution of particles round their axes, at the moment when the body becomes fluid or aeriform, or from the loss of rapidity of vibration, in consequence of the motion of the particles through greater space".
At the present day, as in the time of Davy, we suppose that heat must be the consequence of the movements, vibratory, rotary, etc., of the particles of matter. All researched on the structure of atoms have justified the existence of the movements. Every atom is now compared to a solar system. Naturally we never observe these movements, and mechanical considerations alone lead us to suppose them identical with those of the planets around the sun.
Every component particle of these atoms must be animated by two movements: (1) rotation of the particle on itself; (2) Revolution round a center. These movements may vary, as may the speed of translation, and also the diameter of the orbit traversed. By compelling the molecules to move nearer to or farther from each other, they explain the dilation of bodies by heat and their contraction by cold.
The variations of equilibrium by these elements in motion should produce magnetism, electricity, and heat, but we are entirely ignorant of the way these forces are generated.
We are able to measure heat without knowing anything of its essence. The expression "a quantity of heat" constitutes an arbitrary notion representing the measurement of an effect the cause of which is unknown. "It is nothing else", writes M. Duhem, "than the measurement given by the calorimeter, and is not otherwise defined. The quantity of heat which is disengaged in a modification is, by definition, a quantity proportional to the weight of water which this modification would raise from the temperature of zero to that of one degree". The insufficiency of such a conception is evident.
Physicists have at length, however, laid aside this problem of the causes of heat, and, without inquiring how movements can be transformed into heat, they have confined themselves to endeavoring to determine their nature. Although the problem has been taken in hand by such physicists as Clausius and Helmholtz, no success has crowned their efforts. Assimilating, to simplify matters, the elements of bodies to material points in motion, they have admitted that the average force of this movement was proportional to the temperature, and have endeavored to deduce from it the laws of thermodynamics, and, notably, the principles of Carnot, by means of the theorems of mechanics. It is pretty much generally admitted nowadays that this attempt has completely failed. It has furnished, moreover, no sort of hint either as to the variations of trajectory described by the particles of bodies, according to their solid, liquid, or gaseous state, not on the results of their reciprocal actions.
The early physicists regarded the problem in a much simpler way. For them it was a fluid impregnating all bodies and disengaging itself by combustion. This theory, called the phlogistic, was much shaken when Lavoisier proved that, far from losing weight by combustion, bodies on the contrary gained it. Yet at the time of Carnot, heat was still considered to be a fluid that bodies could part with or absorb, and only differing from the old phlogiston by its imponderability.
In the long run physicists gave up the idea of a caloric fluid; but after taking infinite trouble, for more than 50 years, to substitute the mechanical theory of heat produced by movement for that of the conception of a fluid, they seem now -- in a rather roundabout way -- about to return to the latter. As Prof de Heen has very justly remarked, "The old idea of mixing the phlogistic fluid with matter is identical with the one currently accepted, which consists in mixing with matter electric corpuscles". There is as much reason for imagining atoms of heat as there is for imagining atoms of electricity.
The ancient idea that heat was a kind of fluid has been very fertile. Without it, Sadi Carnot would, perhaps, never have thought of comparing the flow of heat to that of a liquid, and, no doubt, would never have discovered the principle which bears his name and has so deeply modified the direction of the sciences of physics and chemistry.
It must be fully recognized, moreover, that if physicists and chemists reject the idea of assimilating heat to a fluid, they nearly always treat it as if it really were one. Chemists constantly speak of heat as absorbed or liberated by a body. According to them, when a combination is formed it should keep this heat indefinitely until it is destroyed. It then gives it up in quantity exactly equal to that absorbed. Physicists, for their part, tell us that when a body is heated it absorbed heat and restores it as it cools. We should express ourselves no otherwise if heat were really a fluid.
The mathematicians themselves often employ similar language. All their formulas have been framed as if heat were constituted by a fluid. Laplace, Poisson, Lame, etc., assimilated caloric to an expansive fluid, and the temperature of the particles to the tension of the fluid in them. The variations of heat were explained by changes between these particles of caloric proportional to the difference of their respective temperatures. At the present day, when heat is considered to be a vibratory movement of the particles of matter, we often still continue to argue as if it were a fluid, of which it possesses, in fact, many of the properties.
"The analogy existing between the propagation of heat in athermanous bodies and the filtration of fluids through porous masses is so close", writes M. Bousinesq in his Theorie Analytique de la Chaleur, "that we might seek to obtain from it a mechanical theory of conductivity if there were such a thing as a caloric fluid".
We are not certain, moreover, that this fluid does not exist. Electrons are beginning to be made to play a great part in calorific phenomena. After having brought us back to the old electric fluid, they are perhaps going to revive the caloric fluid. For the moment our ignorance on this point is complete.
2. Changes of State of Bodies Under the Influence of Heat and Variations of Energy Resulting Therefrom ~
The effects of heat on matter are of daily observation. The simple examination of the movements of the thermometer column shows that bodies dilate with heat and contract with cold. The sensitiveness of matter is such, that a variation in temperature of the millionth of a degree suffices to modify its electric resistance in a fashion appreciable by experiment. The slightest oscillation in the ether causes it to vibrate and radiate. There is thus a continuous exchange of energy between matter and the ether.
It is no longer possible at the present day to consider matter independently of its surroundings. The variations of the latter regulate its equilibria and also its form, rendering it solid, liquid, or gaseous. Matter corresponds to a state of equilibrium between its internal energies and the external ones which surround it.
The movements of rotation and of revolution of the elements of the atoms unceasingly vary under the action of heat. It modifies not only their speed of rotation, but also the diameters of the orbits traversed. When these increase, the particles of bodies move farther and farther apart, the molecular attractions which constitute cohesion are overcome, and matter passes first into the liquid, and then into the gaseous state. During these changes, bodies absorb determinate proportions of heat which they restore in absolutely equal quantity when they return to their primary state. The energy absorbed by matter being then exactly given back, we should be justified in believing that matter has never either created or destroyed it.
Heat may, from the physical point of view, be defined as a mode of energy producing the change of volume of bodies, and therefore their dilation. This dilation represents an excellent means of measuring it, but the thermometer, which is based on this property, can only indicate a small part of the heat supplied to a body. When, for example, we heat matter to make it change its state -- that is, to liquefy it -- we produce three effects, of which only one is revealed by the thermometer: (1) we increase its temperature; (2) we change the internal disposition of its molecules -- that is to say, we affect an internal work which is not revealed by the thermometer; (3) we change its volume -- that is to say, we effect an external work against external pressure, which, again, is not revealed by the thermometer. It is therefore only a part of the heat produced which has served to change the temperature of the body.
We can, on the other hand, cause the temperature of a body to vary without supplying or abstracting heat from it. This is observed in the operations called adiabatic, for instance, when a gas is compressed in a receptacle impermeable to heat. The temperature is increased by the transformation into heat of the work affected.
The caloric energy necessary to compel bodies to change their state is considerable. To transform ice at 0° C into water at the same temperature, as much heat must be given it as would raise by one degree 80 times its weight of water, or 80 calories. If the water again freezes, it restores the heat absorbed. To transform water at 100° C into steam of the same temperature, the necessary work is more considerable still, since this transformation requires 537 times as much heat as would raise the same quantity of water one degree. As before, these 537 calories are easily restored when the molecules draw together to pass again into the liquid state -- that is to say, when the steam at 100° C condenses into water likewise at 100° C.
To show the magnitude of the energies thus displaced, Tyndall gives the following examples: -- The heat resulting from the combination of 1 kg of hydrogen with 8 kg of oxygen would raise by 1° C the temperature of 34,000 kg of water which corresponds to more than 14 million kilogram-meters. The condensation in water of the 9 kg of steam formed by this combination, represents a work of more than 2 million kilogram-meters. If, by continuing to run down this scale we bring the water to the solid state by lowering its temperature, it would still produce more than 700,000 kilogram-meters.
The figures representing the forces necessary to modify the molecular states are evidently considerable when judged by our usual units of energy, but they are immensely feeble compared with the intra-atomic forces of which we have elsewhere studies the magnitude.
We must bear in mind from what precedes the constancy of the figures representing the caloric energy displaced in the different variations of the state of matter. That which it absorbs in order to pass from one state to another is always rigorously given back when it returns to its first state. There are, then, simple displacements of energy without destruction or creation.
This fact, so constantly observed, seemed a very solid argument in favor not only of the conservation of energy, but also of the important notion that matter and energy are two very distinct things, the first coming as a support to the second, but never creating it.
My readers know that these principles have been overthrown. Practically, however, the ancient notions retain all their values. For, if matter is an enormous reservoir of energy, and is able to disappear by transforming itself into energy, we do not yet know how to extract from it any but insignificant quantities of this last.
3. Can Heat Serve as the Measure of All Forms of Energy?
In all the changes of state of bodies, we have spoken solely of the heat absorbed or liberated, without troubling ourselves about the other forms of energy. Formerly these were ignored, but the deeper study of the laws of electrolysis having shown that the majority of chemical changes are accompanied by the production of a rigidly constant quantity of electricity for each reaction, it follows that these reactions can be expressed in units of electricity quite as well as in units of heat. The tendency of the present day is to measure reactions by the quantity of electricity displaced rather than by the quantity of heat brought into play. The generation of heat and electricity proceeds by nearly parallel steps, so that we may ask ourselves whether these forces may not be secondary manifestations of unknown energies of which we only perceive the transformations. Chemical energy, for example, is perhaps as different from the electricity and the heat it generates as these last are from friction which can also generate them.
Heat being very early known, and all forces appearing able to transform themselves into heat, it was natural to take it as the unit of measurement. When radiations are allowed to fall on an absorbent surface, we consider those equivalent which produce the same amount of heating. In this way the division of energy in the luminous spectrum has been studied. But it now appears that some very active energies can be manifested under other conditions than heat, and cannot, in consequence, be measured by it. The temperature becomes less and less as we advance towards the extreme ultraviolet in the solar spectrum, and ends by being so minute that it is only perceptible to instruments of excessive sensitiveness. If we confined ourselves to caloric measurements it might be said that energy is almost nil at this end of the spectrum. Now, it is, on the contrary, extraordinarily active, for it dissociates the most resisting bodies, and transforms them into a torrent of particles of the family of the cathode rays.
There are therefore forms of energy which cannot be reduced to heat, and which in consequence heat cannot help us to measure. This very important point will certainly someday attract the attention of physicists.
4. The Conception of the Absolute Zero ~
The movements of the particles of heated bodies are communicated to substances in contact with them, and cause their volume to change. It is on this fact that the thermometer is based. Plunged into a more or less heated medium, it indicates the difference of temperature between this medium and that of the melting of ice taken as zero during the graduation of the instrument.
This zero is evidently a very arbitrary one, since we might have taken as the starting point of the graduation the point of fusion of any body whatever. All our zeros -- such as, for instance, that of electric tension -- are equally conventional starting points.
Physicists, however, have for a long time been led to conceive for heat a zero which does indeed deserve the name of absolute which is given to it, since the bodies brought to this temperature would no longer retain any caloric energy. This conception was formed at the time when heat was considered a fluid. The temperature at which bodies would expel all their provision of this fluid constituted the absolute zero.
The theoretic discussions enabling it to be fixed have been numerous. Laplace and Lavoisier placed the absolute zero between 1500 C and 3000 C below melting ice. Dalton fixed it at 1500° C. The reasons for these different conclusions were, however, very unconvincing.
Although it has been abandoned, the theory of the materiality of heat has continued to weigh on the minds of physicist. Considerations drawn from the study of thermodynamics have led Lord Kelvin to adopt for the absolute zero the figure of -273° C, already deduced from the consideration that, as gases contract by 1/273 of their volume per degree, at 273° below the ordinary zero they could contract no further.
According to the conception of the absolute zero, bodies would at -173° C contain no more heat. If heat be only the consequence of the movements of the particles of matter, as is generally admitted, these movements would cease at absolute zero. With this cessation would also disappear, no doubt, the other forces, such as cohesion. One does not, then, very well see what would become of matter. Several physicists, however, at the present day consider the absolute zero as a theoretical and unattainable limit which is merely a datum for calculations.
This theory is much earlier than the date of the discovery of the existence of intra-atomic energy. We may suppose, in strictness, that relatively weak intra-molecular energies may disappear at a certain temperature, but it is impossible to imagine the vanishing of intra-atomic energies. They are so considerable, in fact, that to annul them would require force immeasurably superior to any we have at our disposal. If by any means whatever, such as lowering of the temperature, we succeeded in profoundly disturbing the internal equilibria of the elements always in vibration and rotation, of the atoms of a fragment of matter, they would be disaggregated and would return to the ether.
In this chapter, devoted to the study of heat, we have not had to trouble ourselves with the sensation designated by this term. "That which to our sensations is heat", says Locke, "is objectively only movement". The physicists study these movements, but without having yet succeeded in explaining them. Heat is a chapter of physics of which a few fragments are precise, but which is chiefly composed of uncertainties. We shall see the number of these increase when studying the relations of the movements of matter produced by heat with those ethereal ones which these movements generate.
Transformation of Movements of Matter in Vibrations of the Ether -- Radiant Heat
1. Nature of Radiant Heat -- Absorption and Transformation by Matter of the Vibrations of the Ether ~
The classic term of radiant heat is one of the most erroneous in physics, notwithstanding its apparent accuracy. If we draw near to a fire, it warms us; it therefore radiates something. What can this something be, if not heat?
It took a very long time to discover that a heated body does not radiate anything resembling heat. It is now known that it produces vibration of the ether, which, in themselves, have no temperature, and that it warms us at a distance because the vibrations of the ether generated it by being affected by the molecules of the air or the bodies before it, generate heat. These vibrations are not heat, but simply a cause of heat, as is any movement whatever.
This confusion of radiant heat with the heat of bodies, which the textbooks still perpetuate, for a long time prevented us from recognizing the identity of radiant heat and light, formerly considered to be two different things.
That which we call by the very improper name of radiant heat has for its sole origin the vibrations of the ether. These can produce heat when their movement is destroyed, as does a stone by its impact, but they do not possess, I repeat, any temperature of their own. This is easily proved by interposing a lens of ice in the path of a pencil of radiant heat. However intense the pencil may be, the lens is not melted, while a piece of metal placed in its focus will become incandescent. The ether having no temperature, and the ice being very transparent to its vibrations, they have passed through the ice without melting it. As the metal, on the other hand, stops these vibrations, it becomes incandescent by absorbing and immediately restoring them under the form of other vibrations, becoming in its turn a source of that radiant heat without temperature, the effects of which have just been pointed out.
Since the vibrations o the ether, called by the name of radiant heat, can only produce heat after their absorption by a body, it is evident that in the celestial spaces, where an atmosphere like that surrounding the earth does not exist, an absolute cold must reign in the neighborhood of incandescent stars, such as the sun. The thermometer dipped into these spaces would, however, mark there a very high temperature, because it would intercept the vibrations of the ether. The temperature recorded by it would not be that of the ambient medium, but its own temperature. Ice would not melt, because it allows the vibrations of the ether to pass without stopping them. Metal would become incandescent, because it absorbs the same vibrations.
Life is only possible on our globe by reason of the absorption of the vibrations of the ether by the atmosphere and the earth; if these last were transparent to them, a very intense cold would reign on the surface of our planet.
All the chemical reactions which take place in the interior of vegetables, notably the transformation of carbonic acid into carbon, have their origin in this absorption.
The vibrations of the ether when absorbed by a body may be retained by it, and become the origin of various chemical transformations. They are thus fixed until the time when, by decomposing the body -- that is to say, by bringing it back to its former state -- we make them reappear under the form of heat. We have here one proof the more of the intimate relations of the ether with the matter, and of the exchanges of energy of which it is the seat.
If the vibrations of the ether absorbed by matter are not used in chemical transformations, they only raise the temperature of bodies, and disappear by radiation with a rapidity dependent on the structure of these bodies or of the substances with which they are covered. A vessel of polished metal loses its heat by slow degrees, and this is why we employ it to keep liquids at a high temperature. The same metal, if covered with lacquer, on the other hand, rapidly parts with its heat. These are facts long known, which Lister in other days put in evidence by his cube full of boiling water, the faces of which were composed of different metals. Each face radiated different quantities of heat.
All these facts find a rudimentary explanation in the phenomenon of acoustic resonance. A tuning fork insensible to the most violent noise will vibrate if struck by sound waves of suitable periods. It will even be able to pick out these sound waves from a mixture of very dissimilar sounds. It is therefore sensitive to some and insensitive to others. It is the same with bodies struck by radiant heat. They only absorb certain vibrations and let others pass by them. I shall return to this point in the next chapter.
2. Permanence of the Radiation of Matter ~
Until the absolute zero is reached, matter unceasingly sends vibrations into the ether. A block of ice may therefore be considered as much a source of heat, and for the same reasons, as a fragment of glowing coal. The only difference between them is in the quantity radiated. The frozen plains of the Pole are a source of radiant heat like the burning plains of the Equator, and if the sensitiveness of the photographic plate were not so limited, it would be possible in the very darkest night to reproduce images of bodies by their own radiations, when refracted by the lenses of a camera obscura.
Naturally, these radiations, which all bodies constantly emit, only act on the thermometer when it is plunged into a medium colder than itself. If the instrument is first placed in a refrigerating mixture, capable of lowering the column of liquid to a level corresponding to -50° C, and then placed in front of a block of ice at 0° C, the heat radiated by this block will raise by 50° -- that is to say, will bring back to zero -- the temperature of the instrument. But if this last already marks zero -- evidently no movement of the column of liquid can reveal the radiation. The ice would continue radiating on the thermometer and the later on to the ice, but they would only be exchanging their radiations. The radiation would therefore nonetheless go on, though marked by this exchange. When we say that a body becomes cool by radiation, we necessarily imply that it is plunged into a medium with a lower temperature than its own. Receiving from the latter less heat then it imparts to it, its temperature is lowered until that of the two bodies is equal.
When we are obliged to keep at a low temperature a body which is to be placed in a medium of higher temperature, we surround it with substances impermeable to radiation, and thence called athermanous. Wool and furs possess this property. Pictet has shown that for temperatures below -70 C the majority of athermanous bodies lose their properties and become diathermanous. We can only keep air liquid by enclosing it in double-walled vessels, between the walls of which a vacuum is made, and the inner surface of which is silvered [Dewar flask]. These vessels can also be used to keep liquids very hot, since they prevent the absorption as well as the emission of radiation.
3. The Electric Emissions Which Accompany Heat ~
We have just seen that matter is always absorbing and radiating. The exchange between it and the ether never ceases. The vibrations of the ether intercepted by matter are subjected by it to various transformation of a mechanism unknown to us, and of which we only perceive the extreme terms.
I have never ceased to insist in this and my preceding work on the relations of the ether with mater. They again appear when we examine the electric phenomena accompanying the caloric variations of bodies.
Physicists have had for a long time an inkling of the kinship between heat and electricity, and recognize more and more that the production of the one is accompanied by the simultaneous manifestation of the other. A body which is subjected to friction generates both heat and electricity. The heat which is propagated throughout a wire by the simple twisting of it on itself, generates electricity. A substance which liberates heat when combining with another, liberates electricity at the same time.
It is known also that the electric and caloric conductivities are sensibly in the same ratios for all metals. Those which are good conductors of heat are the same for electricity, and conversely. The chief difference lies in the speed of propagation. Immense in the case of electricity, this is, on the contrary, very slow in that of heat.
If heat easily transforms itself into electricity, the latter no less easily transforms itself into heat. It suffices to pass a current through a metal wire to see the latter become more or less red-hot according to its resistance. If a current be sent through a conducting wire half platinum and the other half silver, the platinum becomes white-hot, while the silver wire, a tenfold better conductor -- that is to say, offering less resistance to the passage of the electricity -- remains dark.
The recent researched mentioned in my last book make it possible to follow much further the course of this analysis. We now know that when, by one means or the other, a body is made incandescent, it emits not only radiant heat and light -- which are, moreover, exactly the same thing --but, in addition, torrents of electric particles. We have even reached the point of admitting -- an hypothesis which the experiments of Zeeman seem to confirm -- that a flame consists only of electric particles in vibration. The movements of these electrons, propagated in the ether, would generate radiant heat and light. It is, however, very possible that the liberation of electric particles which accompany incandescence and many other chemical reactions, is only in many cases a secondary phenomenon, a king of unutilized excess of energies employed in modifying the equilibria of matter.
A constant relation ought to exist between the intra-molecular and intra-atomic energies. Atoms represent the stones of which the molecular edifices are built. In all the operations of ordinary chemistry, we simply displace these stones, and this is, no doubt, why the quantities of heat or electricity then brought into play are always met with again. When by various means, very inadequate as yet, we touch the structure of the stones of the edifice -- that is to say, of the atoms -- we liberate, in the form of heat, or electricity, quantities of intra-atomic forces of which the magnitude will vary according to the disturbances of equilibrium produced, and may bear no relation to the causes of such changes.
The whole of this and the preceding chapter are, if looked upon as explanations, evidently insufficient. Notwithstanding all the formulas with which it bristles, this region of physics is extremely obscure. The problem of heat is one of the most difficult, because its solution demands the knowledge of things which are as yet very difficult of access.
Transformation of Matter into Light
1. The Emission of Light by Matter ~
Light is produced by vibrations of matter propagated under the form of waves in the ether. When these waves possess a length suitable for impressing the eye, we give them the name of visible light. We call them invisible when the retina, which is only impressed by a small part of the whole extent of the solar spectrum, remains insensitive to their action.
Whether it is the vibrations producing the sensation of blue or red, or those which are without action on the eye, such as infrared or the ultraviolet, that are in question, they are all of the same species, only differ by their frequency, and all deserve the name of light. From this general definition there springs a first consequence. We ought to give the name of light to the visible or invisible radiations emitted by matter at all temperatures down to the absolute zero as we have seen when studying radiant heat.
Matter, then, is incessantly transformed into light at all temperatures. An eye with a retina sensitive enough would see in the dark all objects as if surrounded by a luminous halo, and darkness would be unknown to it. Such an eye perhaps does not exist, but different instruments allow us to make a substitute for it.
Let us examine some of the conditions of the transformation of matter into light.
When we heat a body, the vibrations of its particles become more rapid, and its emission of ethereal waves increases. These waves, at first too long to be perceived, as they get near 500° C are short enough to become visible and to give the sensation of red. From 800° to 1000° C still shorter waves appear, and the radiations emitted comprise the whole length of the spectrum. Their slight amplitude alone prevents us from perceiving them. Temperature acts especially by increasing the amplitude of the waves emitted, which renders them visible.
At each temperature, the heated body emits waves different in length according to its nature. The brilliancy of flames depending at equal temperatures on the ratio between the long and short waves emitted by the incandescent body, those sources of light which emit many more of the second than of the first will be the more luminous. The brilliancy of the Auer mantle is due to the weakness of its emissive power in the red and the infrared compared to its power of emission in the visible spectrum. The temperature (about 1650° to 1700° C) does not in this case differ notable from that of a simple gas burner.
As regards the brilliancy of the light, there would be no advantage in raising too much the temperature of a body, because we should then produce, as is the ease with the electric arc, invisible ultraviolet rays. The more, in fact, the temperature of a source of light is raised, the more the radiations it emits are displaced towards the ultraviolet.
The radiation of bodies generated by heating is produced by all actions capable of increasing their vibrations, especially those chemical reactions which furnished the early modes of lighting. As a type, we may quote the combustion of ordinary gas. Formed by a mixture of hydrogen and of carbides of hydrogen, it combines violently with the oxygen of the air when lighted. The particles of carbon from the carbide, being liberated and brought to incandescence, give the flame a brilliancy which pure hydrogen does not possess. Gas therefore owes its brilliancy to these incandescent particles held in suspension. Any solid body whatever -- platinum, for example -- might replace the particles of carbon.
In reality, the phenomena which takes place in any kind of flame -- that of a simple candle, for example -- are quite otherwise complicated. So much is this the case that we might consider a body in combustion, such as a lighted candle, as one of the phenomena in physics most difficult of explanation, and involving the solution, of which we have yet hardly a glimpse, of the problems relating to the dissociation of matter. All incandescence is accompanied, in fact, by the liberation of a torrent of electric particles comparable to the cathode rays or the emissions of radium. This liberation necessarily implies a commencement of that disaggregation of the atom which was formerly ignored, because the provision of energy contained in matter is so immense that the loss of it during combustion then passed unperceived.
This dissociation of the atom in a flame has been made apparent not only by the production of electric particles proceeding from this dissociation, but likewise by the deviation of the electrons of flames by a magnetic field; it has as its consequence the doubling of the spectral rays if the flame acted on by the magnetic field.
2. The Influence of Wavelength and Amplitude on the Action of Light ~
A body thrown into the water produces on its surface a series of concentric circular waves comparable to small parallel hills separated by valleys. The distance from the top of one hill to another is what is called the wavelength; the height of each hill from the bottom of the valley represents the amplitude of the wave. It is the same with light, the sole difference being that the undulations take place in the ether instead of being produced in a liquid.
The length of the wave and its height constitute two very different things which we must keep distinct if we wish to understand certain actions of light.
Whether it is a question of sound or of light, or of any periodical disturbance of any fluid whatever, the wavelength is an element of invariable magnitude during the whole period of a vibration, while its amplitude may vary within wide limits. Waves lose their amplitude by propagation, but their length, and consequently the number of vibrations per second, remain the same. The analogy with the oscillation of a pendulum is complete. Move a pendulum much or little from the vertical line, and the distance it travels in its oscillating trajectory may be very short or very long, but the time taken to effect it will be invariable, and will depend solely on the length of the pendulum.
What is the part played by these two elements, wavelength and amplitude? In the case of the pendulum, the vis viva [kinetic energy] of its waves increases with the amplitude of its vibrations. As regards sound, it is the wavelength that determines the pitch of a given note, while the amplitude determines the intensity of this note.
With light the undulation of the ether give, according to their length, notes which we call blue, red, green, etc. Their length is strictly invariable for each note; but their intensity may vary enormously with the amplitude of the waves emitted -- from 1 to 1 million between 600° and 1800° C, for instance, in the case of red radiations according to the measurements of M. Lechatelier. The intensity of a radiation will make it oscillate between darkness and a blinding flash, without the wavelength undergoing any change.
Besides the temperature, there are different means of increasing or decreasing the amplitude of the ethereal waves, and consequently the intensity of a pencil of light. To do so, it is enough to concentrate or, on the contrary, to disperse it, by lenses of suitable form. The intensity of a note or of a color is then very variable, but the length of the waves which produce this note or color remains absolutely the same during the whole period of the vibrations.
The eye and the ear are not organized so as to accumulate impressions. A color or a note of a given intensity will always produce the same effect, whatever the duration of their action. It is otherwise for certain reagents -- the photographic plate, for instance -- capable of accumulating impressions. We can thus, with a very slight but prolonged intensity, produce effects identical with those obtained with a very great intensity, acting for a very short time. It is the possibility of this accumulation which enables us to photograph bodies having a phosphorescence invisible to the eye, simply because the amplitude of the vibrations of light emitted was too slight to impress the retina.
The sensitive plate sees the radiations emitted because it can accumulate them, and photographs stars which the eye does not see by reason of their too slight amplitude, although the sensitiveness of the retina is enormously superior to that of the plate. The eye is for light what the ear is for sound. There exist dark light and silent sound, which the eye and ear do not perceive, but which suitable reagents may reveal.
From the fact that those stars which are on the edge of visibility take an hour’s exposure to photograph, Deslandres remarks that "the relation between the sensitiveness of the eye and that of the photographic plate should be the ratio of 1/10 second and 1 hour, or (say) 1/40,000".
Whatever be the reagent employed -- retina, photographic plate, or chemical compound -- there is always a minimum of amplitude variable in each, below which light has no action. Berthelot pointed out, for example, that the oxidation of bisulfide of carbon, which in the sunlight can be effected in a few hours, is never effected in diffused light, even in a year’s time. For other reactions, such as the combinations of chlorine and hydrogen, the intensity of light may, on the other hand, be extremely slight. These are phenomena which are not always taken into account, but which must be known in order to understand the effects of light. It is because they have been misunderstood that the variations of certain vegetable functions in the different regions of the spectrum have given rise, as we shall see, to so many contradictory interpretations.
3. The Invisible Spectrum ~
The researches effected during recent years have proved that the invisible solar spectrum is much more extended than the visible. While this last only reaches from 0.4 to 0.8 microns, the invisible spectrum goes a little beyond 5 microns according to Langley [who used a bolometer] -- that is to say, it is about 12 times as long as the other. The invisible spectrum of artificial sources of light is more extended still, since, according to Rubens, it stretches as far as 60 microns.
The plates of the solar spectrum published in textbooks give a very false idea of it, Not only do they reproduce nothing but the visible region, but the distribution of the color in it is very inexact, inasmuch as the prismatic spectra used as models reduce to a fourth or a fifth of its real size the extent of the red, and much exaggerate that of the violet rays.
Figure 14 ~

The distribution of the colors is only exact with the diffraction spectra obtained by means of gratings. The distance between the rays then being proportionate to the wavelength, the red occupies a much more considerable extent than in the spectra obtained with a prism.
It was in fact the employment of prisms for the production of spectra which led us to interpret inaccurately the position of the maximum of caloric energy. It was formerly placed in the infrared. We now know that it is found in the luminous part of the spectrum. But as, in comparison with the total length of this last, the visible region is of very small extent, it follows that the total caloric energy is much greater in the invisible infrared. According to the last measurements of Langley, the visible solar spectrum only contains one-fifth part of the caloric energy of the infrared region. The invisible region of the spectrum constitutes, then, the most important portion of light. It is only the sensitiveness of the human eye which creates the division between the visible and the invisible parts of the spectrum. It is not, doubtless, the same with all animals.
This immense invisible region of the spectrum, wherein is found the greatest part of its energy, ought to play a very important, though hardly suspected, part in the phenomena of vegetable life and in meteorology. We as yet know none of its properties except the caloric action. Its variations probably have considerable effect in the changes of the seasons. Langley has recognized that the solar spectrum changes at the different periods of the year, and that the distribution of its energy is not the same at different seasons.
The one-fifth of the solar radiation which appears under the form of visible light seems at first a very small proportion of the whole. It is in reality very great if we compare it with that of artificial light. According to Wedding’s latest researches (1905) all artificial sources of light, including the electric arc, utilize hardly 1 percent of the radiations produced.
Figure 15 ~
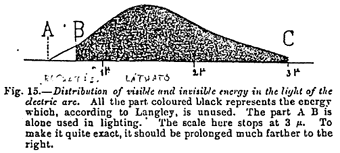
99% of the radiations emitted are, then, invisible.
Although these figures vary with the observers, none of them have found a less loss than 90%. If, then, we estimate at 4 million pounds, as has been done, the annual expenditure on artificial light of a great country like England, we shall see that the discovery of a means of transforming the invisible caloric energy into visible light would effect a saving of nearly 3 million pounds a year for one country alone.
The problem does not appear at all insoluble, since nature has already found the solution of it. The light of phosphorescent animals is almost exclusively composed of rays belonging to the visible region of the spectrum. All phosphorescent bodies also produce light without previous heating. It is probable that in this case, then, it is the energies of the atoms and not the disturbances of the molecules -- as in the case of incandescence -- which come into play. We will return to this point when studying, in a future chapter, the phosphorescence of gases.
4. The Distribution of Energy Throughout the Spectrum ~
The distribution of energy in the various regions of the spectrum has been the object of numerous researches. They have not led, however, to any very useful results, for the simple reason that, at a uniform temperature, the intensity of the various radiations varies greatly according to the source of light. We have seen, for example, that the distribution in the spectrum of a gas burner and in that of an Auer mantle was very different, although their temperature was almost identical.
Nor is there any profit to be drawn from the published researches on the distribution of energy in the solar spectrum, by reason of the very great variation which takes place in the infrared according to the days, the hours, the attitude, and the absorption exercised by the greater or less quantity of water vapor in the atmosphere, etc.
All the early measurements of the energy of the spectrum were, moreover, affected by errors due to the employment of the prism to separate the various radiations. As the prism heaps together the radiations in the infrared, and spreads them out considerably at the other end of the spectrum, it was natural that the heat should be very great in the part where the radiations were most condensed. We therefore supposed that in the infrared was to be found the hottest part of the solar spectrum. This error and the curve which represents it still figure in most elementary treatises on physics.
As soon as we succeeded, by means of gratings, in producing spectra in which the deviation of the radiations is proportional to the wavelength, it became evident that it was not in the invisible infrared, but in the most luminous part of the spectrum, that is from A to D, that the maximum of caloric action in the light of the sun is to be found.
No part of the spectrum is really devoid of caloric action, as physicists for a long time believed. It would suffice to give to any radiation sufficient intensity to cause it to produce any caloric action one could desire.
Independently of the causes of error just enumerated, there is one much more serious still, which is connected with the very principle of the materials used.
Physicists were led to measure the energy of the spectrum solely by the evaluation of the caloric notion of its different parts. M. Jamin (Physique, 4th edition, t. iii, p. 160) shows in the following passage the mental process involved in that conception: --
"It was formerly supposed that three distinct agents emanated from the sun -- heat, light and the chemical rays -- and that each of these gave rise to a spectrum partially superposed upon the two others, but as distinct in its nature as in its properties. But we have been led by the force of events to reject this complicated hypothesis, because all experiments proved powerless to realize in practice the separation supposed to be possible in theory. Everybody now admits that the sun sends us vibrations, which are all of the same nature, and which are only distinguished from each other by their wavelengths. These different actions (luminous, chemical, and caloric) are all executed at the expense of the energy of the vibrations, but the caloric action supplies the only rational measurement of them".
This mode of measurement was applied not only to light, but to all forms of energy. It is deduced from the idea that all the modes of energy, being capable of transformation into heat, are measurable by their caloric effects evaluated in calories or kilogram-meters, which are consequently their equivalents.
By considering in the spectrum nothing but heat, we were naturally led to attribute to it all the actions observed. This is exactly what the last-quoted author did. "It is at the expense of the calorific energy of which the radiations can dispose", he says, "that the impression of light on the eye and that on the photographic plate are alike produced". If it were really so, the rays which can produce the maximum of heat ought to be those which can best act on the photographic plate and on the eye. Now, it is just the contrary which is the case. It is not only in the photographic action that we notice this want of parallelism between the caloric intensity and the effects observed. It is really striking to see produced in very energetic fashion, in the ultraviolet, the caloric action of which is almost nil, certain effects, such as the dissociation of matter, while they are insignificant in the hotter parts of the spectrum.
We ought to conclude from this, that the various regions of the spectrum possess actions having no common measure. According to the reagent employed -- the eye, the photographic plate, the thermometer, the electrometer -- the distribution of energy will be very different. The reagents strongly impressed by a certain radiation are silent to another.
In reality, a curve is required for each of them, and it must not be claimed that the energy of the spectrum can be determined by a single one, as has hitherto been done.
5. The Absorption of Light by Matter ~
To its property of emitting luminous rays, matter adds that of being able to absorb them or to allow them to pass through it. There is thus a permanent interchange between matter and the ether, as I have already pointed out.
When a body allows light to pass through itself it is called transparent. In the contrary case, it is called opaque.
Our ideas as to transparency and opacity have been much modified during the last few years. It is known at the present day that there is no body entirely transparent to all radiations. A strip of glass one-tenth of a millimeter thick, completely transparent to the eye, is yet absolutely opaque for the whole ultraviolet extremity of the spectrum and for a notable part of the infrared.
Transparency is always selective and, consequently, never complete. If there existed a body entirely transparent, it could be exposed to the most intense source of heat without becoming warm, since it would not absorb any radiation. The rise in temperature of a body exposed to a radiation is only due, in fact, to the absorption of it of those radiations which have no temperature of their own.
The greater the opaqueness of a body the more it absorbs and the more it becomes heated, except, naturally, in cases where, through the polish of its surface, it sends back into space the vibrations of the ether which reach it.
We now attempt, as said before, to explain the transparency and opacity of bodies by a phenomenon resembling that of acoustic resonance. A slight modification of the current theory will suffice to show that these phenomena are the consequences of the same law. Matter may be considered to be composed of small molecular tuning forks capable, like ordinary ones, of vibrating to certain notes, but not to others. When struck by the vibrations of the ether, they vibrate according to their structure, in unison with certain vibrations and receive no impressions from the others. The radiation which causes them to vibrate remains, in issuing from the transparent body, exactly the same as when it entered, having undergone, in the case of other modifications, only a slackening of speed, in consequence, no doubt, of the time necessary to increase the vibrations of the atoms.
Opaque bodies must, on the contrary, be formed of elements unable to vibrate in unison with the vibrations which strike them. They can, then, only emit irregular vibrations which vanish at once by transmitting themselves to the neighboring molecules. From these movements must result the heating of bodies struck by light. Absorption would be, then an absolute transfer of the movement of the ether to the bodies which are plunged therein.
When a substance is transparent to one radiation and opaque to another -- which is the general case -- the molecules vibrate in unison with the vibrations which pass through them and absorb others. A red or blue glass possesses its color because it can only allow the radiations of the spectrum corresponding to the blue or red to pass and can keep back the others.
This theory of resonance is only maintainable if we suppose the molecules of bodies to be already animated by rapid movements to which the vibrations of the ether do but impart direction. It would be, otherwise, quite impossible to suppose that the vibrations of the ether could give to the atoms the enormous total of energy necessary to cause them to oscillate with the rapidity of light.
According to this theory, the only difference between a transparent and an opaque body rests on the nature of the vibrations they each emit. The vibrations falling upon them pass through the transparent body, and cause in their passage the atoms of matter to vibrate with the incident ray. They would equally cause the atoms of the opaque body to vibrate, but would diffuse themselves throughout its mass. In both cases the luminous energy, having struck the face of an opaque or a transparent plate, necessarily reappears on the other side. In the case of transparency, the ray on issuing is the same as on entering; in the case of opacity, the plate is heated, and then emits in all directions -- and no longer in one single direction -- radiations with a wavelength greatly differing from that which struck the other side. While the light does not modify the temperature of a transparent body, it raises, on the contrary, that of an opaque one.
If the amplitude of the luminous waves striking an opaque body be great enough, the molecules of this body may be driven sufficiently apart to cause it to pass into the liquid or gaseous state.
The absorption of light by matter is closely connected with the structure of this last. The modifications produces by its changes enable the composition of bodies to be ascertained. They are easily noticed by interposing the substance it is desired to examine between a luminous source and the prism of a spectroscope. Many liquids very transparent to the eye present bands of absorption which vary with the slightest changes in their composition. Traces of impurity are thus easily discerned. It is possible, for instance, to detect the presence of 1/50,000 part of pyridine in ammonia.
Gases are very absorbent for certain radiations, and very little so for others. He gases of the atmosphere totally absorb all the ultraviolet starting from 0.295 microns, and all the infrared beyond 5 microns. The ozone of the atmosphere also shows itself very absorbent for the ultraviolet, and the accidental disappearance of this region from the line M onwards, observed in my experiments, is perhaps due to the momentary excess of this substance.
It is somewhat difficult in the theory of transparency by resonance, given above, to understand how the same body can be transparent or opaque to regions situated at the extremities of the spectrum. Window glass is quite opaque, not only to the ultraviolet, but likewise to all the infrared region beyond 2 to 3 microns, and consequently to the caloric radiations emitted by bodies heated to 100 C or less.
This partial transparency of matter to the luminous vibrations of the ether should be compared with its complete transparency to the electric or magnetic lines of force pointed out previously, which are also composed of ether, but in a form unknown to us. They are the only elements of which matter is unable to stay the progress. Why does it allow the ether to pass in one form and not in another? I can give no answer to this question.
6. The Chemical and Photographic Action of Light ~
When the vibrations of the ether produced by the heating of matter meet a body, they produce varied effects, which we may divide into three classes.
1. Mechanical Action ~ This is the pressure exercised by radiant energy. Being very slight, it can only be made evident by very sensitive instruments. It may, however, be sufficiently intense to annul gravity in the case of very light bodies. The deformation of comets is attributed to its influence.
2. Dissociating Action on the Atoms of Matter ~ This is put in evidence by the researches set forth in my former work, to which I shall return in a later chapter.
3. Chemical Actions ~ Comprise very different reactions (oxidation, reduction, etc) made use of in photography.
Of these different actions, I shall now only study certain particular effects with regard to photography which have been observed in the course of my researches.
It being conceded that the electric particles of the cathode rays and of radioactive bodies impress photographic plates, we might be tempted to ascribe the formation of the latent image to a kind of ionization of the gelatino-bromide of silver. I formerly thought of maintaining this hypothesis, but there are the two following facts against it: (1) It is impossible to observe any radioactivity during the exposure of the photographic plate to the light; (2) the blue rays which chiefly act on the photographic plate are by no means the most active agents in the dissociation of matter.
The elucidation of this last point led me to inquire which were the radiations with the greatest action on the photographic impression.
In order to make these evident I exposed a photographic plate behind a spectroscope, and watched in which region the impression commenced. It always began in the blue, and never in the violet or the ultraviolet.
Figure 16 ~
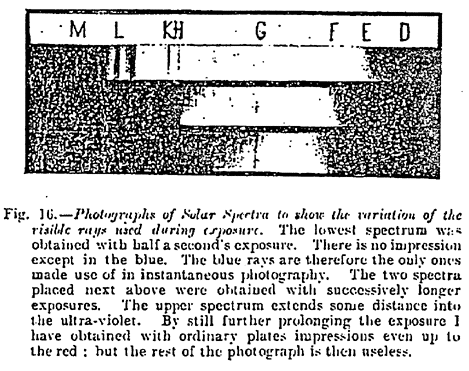
Although the Jena-glass prisms of my spectroscopes allow nearly all the solar ultraviolet to pass, they absorb, however, a portion, and it might be objected to the above experiments that the feeble action of the violet and the ultraviolet was the result of this absorption. I therefore asked M. de Watteville, who owns a large spectroscope with a quartz prism, very transparent to ultraviolet rays, to repeat my experiments with his instrument. They gave results identical to those set forth above. The impression always begins in the blue, and is only propagated some time afterwards into the ultraviolet. It may be gathered from this that the use of objectives of quartz or of glass very transparent to the ultraviolet would offer absolutely no advantage in instantaneous photography.
It would be much more interesting to increase the sensitiveness of the plates for all regions of the spectrum. The part utilized in photography hardly represents more than about the 20th part of the solar spectrum, which goes from 5 microns to 0.295 microns, including the visible and invisible rays. The visible part only extends from 0.4 to 0.8 microns. Even if we only take note of the visible spectrum, it will be seen that the photographic plate utilizes but a very small part of it.
No doubt, by various means, we can render the plates fairly sensitive as far as the red; but this sensitiveness is very illusory, for enormous differences of exposure are always required to obtain images with blue, green, and red light. The only real advantage of the pates termed monochromatic is that they are less sensitive to the blue than ordinary plates. The same result is obtained by simply placing a yellow glass before the objective. With any plate whatever we obtain an impression as intense as can be desired by sufficiently prolonging the exposure. A landscape may very well be photographed through a red glass.
Figure 17 / 18 / 19 / 20 ~
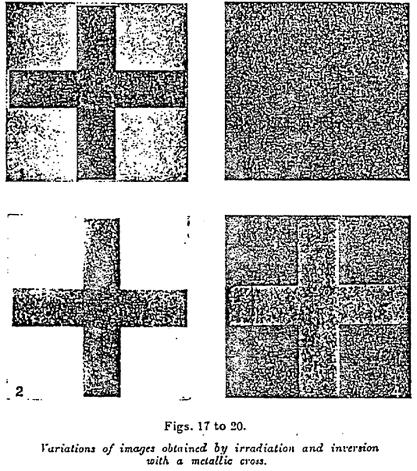
It is this difference of rapidity of action in the different luminous radiations which changes all the values in the photographic reproduction of landscapes. We obviate these differences somewhat by prolonging the exposure so as to allow the feebly actinic rays time to act, but then soon comes in the phenomenon of irradiation consisting in the fact that every part impressed acts as a luminous center on the neighboring region, not only directly, but by its reflection on the posterior of the glass. I have made not a few experiments on this subject, and have observed that with sufficiently long exposures the impression can be propagated to within half a centimeter of the region reached by the light. It is for this reason that the photography of very fine lines is very difficult. Vain attempts have been made to thus reproduce by photography the diamond-cut gratings employed in certain processes.
I will sum up for my photographer readers the experiments that I have carried out -- with the spectroscope described in another chapter -- for the examination of those regions of the spectrum which impress photographic plates according to the length of exposure, the nature of the plates employed, and the colored glasses placed before the objective.
Parts of the Solar Spectrum utilized in photography according to the length of exposure with ordinary and with orthochromatic plates (Cf. Figure 16)
(1) Ordinary rapid plates: Instantaneous exposure -- The impression extends from F to the middle of the interval between H and G; 2-seconds exposure -- The image extends up to K on one side, and nearly to E on the other; 15-seconds exposure --The impression is prolonged beyond L on the side of the ultraviolet, and extends nearly up to D on the side of the red.
It is, then, sufficient to expose an ordinary plate long enough for it to be impressed by the least actinic rays. In proportion as the exposure is lengthened, the intensity of the impression increases between H and E, and much less rapidly from E to A. We then have a plate over-exposed for the blue, and hardly enough so for the other colors.
Influence of a colored glass -- A blue glass reduces but very feebly the image during instantaneous exposures even with the ultraviolet region. A yellow glass does not reduce the intensity of the image on the side of the red, but does so on the side of the blue, i.e., from H to the ultraviolet. If instead of 1 second’s exposure we give it 30 seconds, all the colors impress the plate, and, moreover, as the action of the blue is much extended, the unevenness of the impression is diminished. In photography therefore, as soon as one has to prolong the exposure, a dark yellow glass should be put in front of the objective. The best orthochromatic plate is an ordinary one with a yellow glass. A green glass would further reduce the impression on the side of the blue, but its use would only be of advantage with a too prolonged exposure.
(2) So-called orthochromatic plates: The substances with which these plates are covered render them much less sensitive to the blue rays than ordinary plates. Their sensitiveness extends from a little on the side of the red to beyond D, but without in any way attaining the ray A save with an exaggerated exposure. These plates behave in reality like an ordinary plate before which one has placed a yellow glass, but are very inferior to it.
They are very little sensitive in the green (between E and F), i.e., exactly in that region where sensitiveness is most necessary. Moreover, if their sensitiveness is greater than that of ordinary plates on the side of the red, it is much less so on the side of the violet. If one uses them with a yellow glass, as has been proposed, the effects are disastrous. The impression between E and F, i.e., in the region of the green, from being insufficient is arrested altogether. Orthochromatic plates -- at least those made in France, which I have alone studied -- possess beside the above defects that of being foggy. Even when one develops them in complete darkness they give gray and flat images.
The Dematerialization of Matter Under the Action of Light
1. The Dissociation of Matter Under the Influence of Different Radiations of the Solar Spectrum ~
I have studied at length, in The Evolution of Matter, the dissociation which all bodies undergo under the influence of luminous radiations, and have shown that a body struck by light emits effluves of the family of the cathode rays, of which the quantity varies considerably with the nature of the radiations. If I return to this question, it is because I have been led to study in this work the principal actions of light. My experiments on this subject were recently verified by one of the most illustrious scholars of the day, Sir William Ramsay (1).
[(1) "The work", writes Sir William Ramsay, "was undertaken with the object of repeating some experiments of LeBon, described in several papers in the Comptes Rendus, and afterwards in greater detail in his treatise, The Evolution of Matter. It will be remembered that LeBon, by allowing ultraviolet to fall upon clean metallic surfaces raised to a high potential, caused them to give up their charges". -- Philosophical Magazine, October 1906, p. 401)
He has published, with regard to the dissociation of matter under the influence of light, a memoir extremely remarkable, not only on account of the precision of the experiments, but also of the theoretical considerations which it contains. The results obtained by him were identical with my own, and he entirely admits the theory of the dissociation of matter. His conclusions are even bolder than mine.
"If it turns out to be true", he says, "as Soddy claims to have shown" that a disintegrating element which parts with beta rays or electrons, leaves behind it matter not associated with a positive charge; and if it be also true that such ‘disintegration’ implies transmutation into some other form of ‘elementary matter’, then it may be that the phenomena of which a description is given on the following pages refer to cases of transmutation. When zinc, for example, illuminated by uv light parts with corpuscles, it may be that the residual matter -- the zinc minus electrons -- is no longer zinc, but some other form or forms of elementary matter". Ramsay considers the action of uv light, moreover, as a kind of detonator which produces the disintegration of the elements of matter.
I was very pleased to see so eminent a scholar confirm the correctness of my experiments, and arrive at the conclusions which I have so long upheld. It will not be without interest to state briefly the origin of these last.
The experiments by which I demonstrated that the action of light on bodies produced effluves similar to those of uranium -- the only radioactive body then known -- are not new, since they were published for the first time about 10 years ago. They were the starting pint of my theory of the universal dissociation of matter, and I have recurred to them in several memoirs.
After having shown that solar light exercised in different degrees a dissociating action on all bodies, I commenced the examination of uv radiations, the study of which had given rise to many works. My remarks demonstrated: --
(1) That the so-called negative discharge was likewise positive, contrary to what was then taught.
(2) That the discharge of electrified bodies is very different according to the bodies employed, a point likewise verified by Ramsay, and contrary to what was then taught.
(3) That it was not at all by the pulverization of the metal struck by light that the discharge was effected, as was formerly the opinion of Lenard, but by the dissociation of its atoms. This most important point, but little disputed at the present time, and likewise admitted by Ramsay, was very new and unforeseen at a date when no one dreamed of establishing a kinship of any kind between the effluves produced by the action of light and the cathode and uranium rays.
All these experiments, which appear so simple when one reads them set forth in a book, bristle with enormous difficulties, and, above all, with causes of error, which explain the erroneous opinions formulated by observers. They studied, moreover, the action of light on bodies without having ever suspected that from this study would one day issue the theory of the dissociation of matter.
Other persons will carry on these researches, for the subject is far from being exhausted. I shall render them service by pointing out the causes of error which delayed me for a long time, and finally led me to establish the spontaneous radioactivity of all the metals. Nothing would be more instructive for the history of the evolution of ideas than the recital of the uncertainties through which those engaged in research have passed, and of which their final works naturally contain no traces.
In my first experiment had indeed verified that the effluves emitted by bodies subjected to the action of light passed, as has been likewise recognized by Ramsay, through thin metallic screens. But as they sometimes seemed to transpierce somewhat thick ones, I had to seek the reason of this anomaly. The cathode rays, to which I assimilated these effluves, can, in fact, only pass through extremely thin plates.
I first observed that these effluves went round obstacles in the most curious way, as if they rolled on their surface. The remedy seemed very simple, since it was only a question of giving to the supposed screens the form of a closed cylinder surrounding the ball of the electroscope. But instead of simplifying the question I had created new problems, to interpret which took me several months.
The electroscope, surrounded by its protecting cylinder, on exposure to the sun discharged itself to the extent of several degrees in a few minutes, and then it gave no further discharge, even when the metal was cleaned. If the cylinder was replaced by another of the same substance the discharge recommenced, and then after a certain lapse of time stopped again. For what reasons did a body having certain properties lose them a few minutes later?
I will not enumerate here all the researches made to separate the factors which might be at work and to study the action of each. From one elimination after another, there remained only the influence of heat. This was indeed the active cause, for by replacing the sun by a body heated but not incandescent, and placed in the dark near the cylinder surrounding the electroscope, the discharge took place; but, as with the sun, it soon stopped. What part did heat play in this phenomenon?
Evidently it was possible that an amount of heat only capable of raising by a few degrees the surface of a metal should render the air contained in its interior a conductor of electricity. Heat, moreover, could not be the only element which intervened, since the metal cylinders exposed to its action soon lost their influence on the electroscope. No amount of cleaning restored their properties. The majority of them, however, regained them spontaneously in the course of a few days.
Again I had to proceed by successive elimination, and I at last succeeded in verifying that the metals lost under the influence of heat something which they cold afterwards regain by repose. This something was simply a small provision of radioactive particles formed spontaneously in all bodies. As the final result of these researches I reached the two following conclusions: -- (1) Light, especially the uv rays, which only exercise, as is known, an insignificant caloric action, dissociates matter and transforms it into products analogous to those emitted by radium or uranium; (2) outside the action of light, and independently of it, luminous or dark heat provokes in bodies the loss of an infinitesimal quantity of the radioactivity they contain, which may be spontaneously regenerated. All bodies are therefore slightly radioactive, and the dissociation of matter is indeed a universal phenomenon.
Ramsay has very thoroughly observed in his skillful experiments this ‘fatigue’ of metals, which lose their properties more or less after a certain time. He attributes it to a modification of the equilibrium of the atoms on their surface, a theory which, however, does not sensibly differ from mine.
2. Origin of the Phenomena Attributes to the Presence of Radium ~
Since I am on the subject of radioactivity, it will not be without interest to say a few words on a great discussion which has been eagerly followed by the English public, and in which the most eminent scholars -- Lord Kelvin, Sir Oliver Lodge, Sir William Crookes, etc. -- have taken part. From the scientific journals it has passed into political papers like the Times. Though the engagement was sharp, the conclusions have remained very uncertain.
Its starting point was the extension of this theory, still very general but very erroneous, as may be seen by the above statements, that all radioactivity is due to the presence of radium or some body of that family.
Radioactivity being now found everywhere, physicists who do not yet admit the theory of the universal dissociation of matter are indeed compelled to suppose that there is radium everywhere. After having been the most rare body in nature, it should now be the most abundant.
Starting with this idea, a physicist maintained before the British Association that the internal heat of the globe might well be due to the action of the radium of which the earth should be full.
Persons who have not made a deep study of this body may think that it is a well-defined substance like sodium or gold, and that consequently it is easy to ascertain its presence by certain reagents; now, it is nothing of the kind. Let us put aside certain rays in the spectrum of a rather disputable interpretation, and which, moreover, are only observed in very concentrated solutions of salts of radium; and let us examine on what is based the assertion that this body is very common.
It is simply this fundamental characteristic -- the emission of particles which bear a certain quantity of electricity, and are therefore capable of discharging an electrometer. There exists no other means of practical investigation, and it was by taking this exclusively as a guide that radium was finally isolated from the various substances with which it was mixed up.
This characteristic would, moreover, be an excellent touchstone if radium or the substance of the same family were alone present in it. But all bodies in nature possess it, as I have shown, either spontaneously or under the influence of very varied causes, such as light, heat, chemical reactions, etc.; and it follows that properties are attributed to radium which may belong to very different bodies. If we are bent on admitting that radioactivity is the cause of the internal temperature of the globe, there is no need to invoke to supposed presence of radium. All bodies at a high temperature, such as are apparently those which exist in the interior of our planet, liberate torrents of electric particles analogous to those produced by radium. I do not know whether they serve to maintain the earth’s heat, but it seems more reasonable to believe that they play a part in the production of earthquakes.
That which concerns the actions of light on matter may be summed up in the statement that the light absorbed by a body transforms itself, according to that body and to the rays which act on it, into very different effects -- light, heat, chemical equilibria, dissociation of matter, etc. In the case of dissociation, the energy emitted by the dissociated body in the form of different particles may be far superior to the energy which provoked its dissociation. Light, then, acts like a spark on a mass of gunpowder. It may therefore be said in a general way that all the physical or chemical properties of a body which has absorbed light are more or less modified by the fact of this absorption alone.
The Problems of Phosphorescence
Chapter I
Phosphorescence Produced by Light
1. The Different Forms of Phosphorescence~
The name of phosphorescence is given to the property which several bodies possess of becoming luminous after having been exposed to various influences, that of solar radiations especially.
Phosphorescence, one of the most difficult problems in physics, and one of these of which the interpretation is the most complicated, realizes the apparent paradox of generating cold light -- that is to say, light without any rise in temperature. In all our ordinary sources of lighting, light is only manifested after the bodies producing it have been first brought to a high temperature.
For a long time, phosphorescence was thought a phenomenon as rare in the mineral as in the animal world. Recent researches on deep-sea animals prove that, for an immense number of beings, phosphorescence is a normal means of lighting which enables them to guide themselves in those dark abysses of the sea where the sun never penetrates. It may be asked at the present day whether the animals knowing no other light but phosphorescence are not more numerous than those whose light is the sun. The phenomena of phosphorescence which formerly struck us by their exceptional character, now do so by their frequency.
We have often come across the action of phosphorescence in the course of our researches. The documents already published, which were confined almost entirely to the researches of Edmond Becquerel 50 years ago, not enabling me to explain the phenomena observed, I was led to take up this study anew. The new facts which I recognized are soon stated.
The customary divisions of phosphorescence being very artificial, it would be useless to reproduce them. I shall confine myself to dividing the phenomena into four classes. The three first have long been known. The fourth is due to my researches.
(1) Phosphorescence generated by light ~ (2) Phosphorescence independent of light and determined by different physical excitants such as heat, friction, electricity, and x-rays ~ (3) Phosphorescence by chemical reaction. In this class are placed the luminous phenomena exhibited by certain living beings ~ (4) Invisible phosphorescence. This comprises the production of light incapable of impressing the eye, but able to impress the photographic plate and to be rendered visible by different means.
We will only study, in this chapter, the phosphorescence produced by light.
2. Action of the Different Regions of the Spectrum on Bodies Capable of Phosphorescence ~
Many bodies, either natural, like the diamond, apatite, fluorite, and leucophane, or artificial, like the sulfides of the alkaline earths, have the property of shining in the dark after having been exposed for a moment to daylight.
With the exception of the diamond, of which the luminosity is, at times, very vivid, the phosphorescence acquired by minerals is always much inferior to that of artificially manufactured substances. A very large number of bodies are capable, as Becquerel showed with his phosphoroscope, of acquiring the property of shining for a small fraction of a second after being exposed to light.
In this last class of brief phosphorescence are placed the compounds called fluorescent. It was formerly wished to make of these a special class, on the pretext that they had the property of transforming invisible uv light into visible light. This property is really common to the various phosphorescent bodies. Nearly all, in fact, are capable of being illuminated by the uv end of the spectrum.
The action of the various regions of the spectrum is best observed with the phosphorescent sulfides. These sulfides are only four in number: those of calcium, barium, strontium and zinc. Exposed to the light for a few seconds, they acquire a phosphorescence which lasts several hours after the insolation [exposure to the sun], but constantly decreases during this period.
The great sensitiveness of these bodies to light comes very near to that of the gelatino-bromide photographic plates.
The sulfides of calcium, of strontium, and of barium have very similar properties; they hardly differ from each other, except by the color of their phosphorescence and the rapidity with which they are impressed by light. The sulfide of calcium is the most rapidly impressed, that of strontium the least so. This last requires several seconds of insolation to reach its maximum of illumination, while the sulfide of calcium can be impressed in 1/30 second in the sun.
The sulfide of zinc with green phosphorescence -- and not those with the yellow or orange -- possesses, for the study of the radiations from green to very far in the infrared, a special sensitiveness lacking in the other sulfides. Thanks to this, I was able to demonstrate the great transparency to light of bodies formerly deemed very opaque. A screen of zinc sulfide exposed to daylight when exposed to yellow, green, red, and especially to infrared radiations, is immediately extinguished. By precise measurements effected by diaphragms, of which I had measured the speed with a registering chronograph, I was able to note that, in less than 1/10 second, insolated zinc sulfide began to be extinguished in the infrared. I made use of this property to obtain instantaneous photographs in complete darkness, as will be seen in another chapter.
The extreme sensitiveness of zinc sulfide renders its illumination very variable, according to the proportion of infrared rays contained in the various sources of light. While the other sulfides become illuminated by the simple light of a paraffin lamp, zinc sulfide not only does not do so, but becomes immediately extinguished if it has been previously exposed to daylight.
The sulfides of calcium and strontium being little sensitive to the infrared rays, light up very well, on the contrary, with the light of a lamp or even of a candle. We thus obtain at once the means of distinguishing zinc sulfide if it be fraudulently substituted for zinc sulfide, although its phosphorescence possesses the same green tint. A screen of strontium sulfide becomes illuminated by the light of a candle; a screen of zinc sulfide previously exposed to sunlight, on the contrary, becomes extinguished. I must add that zinc sulfide with green phosphorescence, the only one serviceable for these experiments, is rarely met with in commerce.
If zinc sulfide is a valuable reagent for the infrared rays, calcium sulfide is as valuable for the radiations of the other extremity of the spectrum -- that is to say, for the blue and violet rays. One-tenth second’s exposure to light suffices for its impression to commence.
To study the action of the various rays of the spectrum on phosphorescence, we have only to place in the focus of a spectroscope adapted for projection and mounted on a camera obscura, a plate covered with a phosphorescent sulfide. And after exposure to light to open the carrier in the dark.
I used a 3-prism spectroscope in front of which was a condensing lens, which is necessary in many experiments in order to increase the intensity of the luminous rays falling on the plate. In studying the action of light on phosphorescent bodies, these latter ought to be spread on glass or cardboard screens. The process of making phosphorescent screens, which I finally adopted after trying several, is the following: The sulfide is ground in an agate mortar and passed through a sieve of the very finest silk. It is then thoroughly mixed in the same mortar with a varnish known to color merchants a s bronzing varnish; the proportion of the powder added to the varnish may vary according to the quality of the later, but must not be less than 30 percent. When the mixture is perfectly homogeneous, it is poured, without being allowed to settle and in the same manner as collodion, onto a piece of cardboard laid flat. Care must be taken to gum on the edges of the card a small mount half a millimeter thick, and two or three mm wide.
If the mixture is poured onto a strip of glass, we obtain a transparent screen having exactly the appearance of fine ground glass. If the liquid is poured on cardboard, the screen is naturally opaque, but as the coating can be made thicker, it is more luminous. When too much varnish has not been put into the mixture, the screen is dry and ready for use in 15 minutes.
If we wish to preserve the image of the sulfide impressed by the various rays, all that is needed is to lay the screen for a few minutes on a photographic plate, which is afterwards developed in the ordinary way.
When operating with screens as above described and placed in the focus of the spectroscope nothing is more simple than to observe the action of the various parts of the spectrum. We then remark that the only part of the solar spectrum able to impress phosphorescent bodies starts from the blue to end very far in the uv. The rest of the spectrum -- that is to say, all the rays from the green to far on in the infrared -- are not only without action on the production of phosphorescence, but destroy it when directed on a phosphorescent body made luminous by the uv rays.
These facts had been summarily observed long ago, but the interpretation of them was very incomplete, for the excitation of light was attributed to a caloric excitement which obliged the sulfide to emit its phosphorescence in a short space of time. As far as concerns zinc sulfide especially, comparative experiments made on screens placed one beside the other have shown me that no period of excitement ever precedes the period of extinction. It is only in the case of calcium sulfide that a very slight excitement of phosphorescence is seen to precede its extinction.
From the point of view of its action on phosphorescent bodies, the solar spectrum thus comprises two regions very different, since their properties are quite opposed to each other: (1) a region of illumination corresponding to about half of the visible spectrum, ranging from the blue to the uv; (2) a region of extinction corresponding to the other part of the spectrum and going far into the infrared.
In the part intermediate between these two regions, varying slightly according to the sulfide used, but oscillating round the line F, there is a zone at once extinguishing and illuminating according to circumstances. With a screen of unexposed sulfide, it excites a certain degree of phosphorescence, which is always very slight. With a screen rendered very brilliant by previous insolation, it also restored the phosphorescence to a slight degree.
These last experiments, easy to observe in a camera obscura furnished with a spectroscope, are more conveniently, though less exactly effected, with a simple yellow glass. If, in a photographic slide closed y a light yellow glass, a screen of unexposed calcium sulfide is placed, and beside it another screen previously insolated, and the frame is opened in the dark after the exposure of the two screens to sunlight through the yellow glass, it is observed that they are both very slightly luminous. On the non-insolated one, the rays which are both extinguishers and illuminators have acted as the latter, and have produced a slight phosphorescence. On the screen previously insolated of which the phosphorescence was at first very bright, they have acted as extinguishers and have reduced it considerably. There therefore exist rays possessing the property of both producing a certain degree of phosphorescence and extinguishing it when it exceeds that degree.
The above results are of great importance. They will permit us, in another chapter, to resolve by very simple experiments the question of the existence of the antagonistic action of the two extremities of the spectrum. This point had been discussed for more than 50 years, and photographic experiments have not hitherto thrown much light upon it.
3. Phosphorescence of the Diamond ~
Although by its phosphorescent actions the diamond resembles the bodies we have just studied, it yet possesses special and highly interesting properties, the study of which delayed me sometime. It is for this reason that I devote a section to it.
The diamond differs from the other natural minerals capable of phosphorescence, because while in these last the aptitude for becoming luminous under the influence of light is in part destroyed by heat, in the diamond it is not so.
Although the property possessed by the diamond of becoming luminous in the dark after having been exposed to the light was known throughout antiquity, its phosphorescence has not been the object of any special study. Mineralogists had not even taken the trouble to seek for the origin of phosphorescent diamonds, and to note that while certain mines furnish diamonds which are always phosphorescent, the contrary is the case with others.
Cape diamonds, often as colorless as those of Brazil, and sometimes larger, are always very inferior to the Brazilian, not only by their hardness but also by their brightness. Placed by the side of the Brazilian, they appear dull.
In order not to confuse the particular with the general, I studied about 200 diamonds of all sizes, some Cape and some Brazilian. The latter mostly came from the Bahia mine, and presented all known varieties of color.
In order to study the visible phosphorescence, the diamonds were subjected to an illumination produced by a ribbon of magnesium 15 cm long, et on fire by a spirit lamp. This operation must always be effected by an assistant, while the observer remains in the dark so as not to be dazzled by the strong light of the burning magnesium, which prevents him afterwards from seeing the phosphorescence.
The first trials made on a parcel of about 100 diamonds of all tints, half of them Brazilian and the other half Cape, immediately showed me one curious fact. Nearly all the Brazilian and all those of the Bahia mine were, during the operation, brightly phosphorescent, as much so as an isolated fragment of zinc sulfide. Not one of the Cape diamonds was phosphorescent.
The non- phosphorescence of the Cape diamond is, however, not absolute, for after one has remained in the dark for at least 20 minutes, in order to rest the eyes -- during which time the diamonds should be exposed to the sun by an assistant -- a very slight phosphorescence on nearly half of them is detected. This phosphorescence is on the border of the perceptible minimum of light, and is in no way comparable to the brilliant light of the Brazilian diamonds.
The same experiments repeated many times with other diamonds of known origin have always afforded the same results (1).
[(1) This means of distinguishing Brazilian from Cape diamonds is very exact, and has ore than once enabled me to enlighten purchasers of the real value of their diamonds, indications which have always been found correct by appraisers. I was able to discover immediately, out of a parcel of 60 Cape diamonds, a Brazilian one placed there in error. This diagnosis is available to everyone, and will enable many persons to observe that they sometimes pay nearly double their value for diamonds. A diamond sold for 10,000 francs on the strength of it being Brazilian is in reality only worth 6000 francs if it is from the Cape.)
As a first conclusion, we see that it is not to the coloration of the diamond, but to its geological origin, that its phosphorescence is due. Whitish or yellowish diamonds from Bahia are phosphorescent, while those from the Cape are not, whatever be their color.
As with the various phosphorescent bodies, pulverization notably reduced the phosphorescence of the diamond, but does not destroy it.
All diamonds which phosphoresce by the action of light also do so when a pencil of x-rays is directed upon them.
Subjected to the electric induction spark in the method described in another chapter, all diamonds become phosphorescent. They likewise become so when exposed to the influence of radium, even through a thin leaf of aluminum.
We shall see in another chapter that diamonds likewise exhibit the phenomenon of invisible phosphorescence.
The aptitude of diamonds for becoming phosphorescent by the action of light is not destroyed by heat, as in the case with many mineral substances. While, after being calcined for 15 hours, it was destroyed in the case of many bodies, such as fluorspar, apatite, etc., yet I have been able to heat diamonds to 1000° C for 60 hours without altering their aptitude for phosphorescence. They have then been reduced to an impalpable powder in an agate mortar, and then calcined over again (1). This has not prevented them from again shining after insolation.
[(1) This operation was necessary in order to discover if the phosphorescence were not due, in the case of the amethyst, so foreign bodies destructible by heat, It is not a very economical one, for the mercantile value of cut diamonds of small dimensions is about 1300 francs per gram. This cost would be much reduced by employing rough diamonds, but Brazilian rough diamonds are almost undiscoverable in Paris, merchants finding greater profit in importing them cut.)
This persistence of the aptitude for phosphorescence, notwithstanding so prolonged a calcinations, shows that, if the phosphorescence of diamonds is due to the presence of foreign bodies, these bodies are not altered by heat, or at all events by a heat below that at which the diamond is destroyed.
We are generally taught in the textbooks that the rough diamond is not phosphorescent, and only acquires that property after being polished. Landrin, in his Dictionnaire de Mineralogie, expresses himself as follows: -- "A crystal of fluorite is only phosphorescent when polished. The contrary is the case with the diamond, which only gives light after having been subjected to polishing, and does not manifest this faculty when in its natural crystalline state".
I was almost convinced of the inexactitude of this assertion, since, according to my observations, the pulverized diamond does not lose its aptitude for phosphorescence. I was anxious, however, to verify by experiments this belief of mineralogist; for the scientific consequences of the property they attributed to the polished diamond would have been very great. As I expected, it was one of those classic errors repeated without verification to which repetition at length gives indisputable authority. Having succeeded in procuring some rough diamonds from Brazil, yellow, blue, and transparent -- some crystallized, others rounded -- I was enabled to note their phosphorescence by the action of light.
The phosphorescence of diamonds seems to be connected, as in the case with other bodies, especially the sulfides before mentioned, with the presence of traces of foreign substances. The most transparent diamonds, when incinerated, leave a small quantity of ash hardly less than 2 percent, containing various bodies -- magnesia, lime, and especially iron.
4. Relation Between the Intensity of the Phosphorescence and the Temperature of Insolated Substances ~
Is the degree of phosphorescence which an insolated body may reach, closely related to the temperature of this body during insolation? Experiment alone can decide this question.
At a temperature markedly below 0° C, and varying according to the substance, bodies exposed to the light do not acquire visible phosphorescence. Above this temperature the intensity of phosphorescence obtained by a body exposed to the light increases when heated up to 100° C. Above 100° C, the phosphorescence it may acquire diminishes, and towards 500° C falls to nothing. The influence of this high temperature may be explained by admitting that the precipitation of the light by heat is then as rapid as the absorption. The expulsion taking place at the same time as the absorption, the phosphorescence does not appear.
The above facts can be verified by means of screens of calcium sulfide placed on cards divided into halves. The two halves, placed side by side in the dark, on recipients brought to different temperatures, are examined as soon as they have been lighted up by a magnesium ribbon. Towards about -190° C, which temperature is obtained by plunging the screens of sulfide into liquid air, no phosphorescence under the action of light is observed, as was first recognized by Dewar; but on withdrawing the screen, and leaving it for a moment in the dark at the ambient temperature, it becomes luminous. The passage from -190° C to the ambient temperature represents to the sulfide a considerable increase of heat, which causes it to rapidly expel the invisible phosphorescence acquired at -190° C.
But it is between 0° C and 100° C that the experiment is most easily effected. One half of the screen having been placed on a block of ice, the other on a sand bath heated to different temperatures, we observe after exposure to magnesium light that the sulfide exposed when heated to 100° C is much more brilliant than that exposed to 0° C. In the case of calcium sulfide the difference is considerable, but much less so in the case of zinc sulfide.
If one of the two screens is placed on a plate heated to 200° C, and the other kept at the temperature of the ambient air, i.e., about 15° C, we recognize, after illumination by magnesium, that the screen heated to 200° C is much less brilliant than the other. Repeating the same experiment with a screen heated to 500° C, we only obtain on it an extremely light phosphorescence. I have given above the probable reason for these differences.
5. Loss of phosphorescence by the Action of Time ~
What has been said of the action of various rays of the spectrum and of the temperature during exposure has already proved that the intensity of phosphorescence depends on several factors.
There remains to us one more to study, viz. Time. All authors have regarded it as having a preponderating influence.
Time has, on the loss of phosphorescence, an influence evident but inferior to that of temperature. As the visible phosphorescence which follows insolation hardly exceeds for the most sensitive bodies the duration of a few hours, time has hitherto been considered as the principle element destructive of phosphorescence. This element has in reality no fundamental influence, since, by means of a suitable temperature, we can entirely eliminate its action.
Curves of the loss of phosphorescence as a function of the time have several times been published, and their equations calculated. These curves would only mean something if they expressed the loss at a given temperature, for this loss varies with the temperature. The number of curves would then have to be very considerable, since a special one would be required for each temperature.
Curves thus constructed would have, moreover, no characteristic in common. At a very low temperature varying with each body, the curve of loss as a function of the time would be represented by a line nearly horizontal, which would signify that at that temperature the phosphorescence diminished very slowly. At a high temperature the curve would be almost vertical, signifying that the loss of phosphorescence is, on the contrary, very rapid. At an intermediate temperature the curve would be represented by a line at first and during the first few seconds after insolation almost vertical, and then after a fairly short time almost horizontal.
If the influence of temperature had been more carefully studied, it would have been seen long ago that these curves of the loss of phosphorescence as a function of the time, since they set aside a much more important factor than time, could possess no correctness.
Not only is time a secondary factor in the phenomenon, but a moment arrives when it has no effect on the loss of phosphorescence. We shall see, in fact, that after a certain emission of light at a given temperature, the body retains indefinitely a residual phosphorescence so long as its temperature is not again raised.
This law is very general. The loss of phosphorescence after insolation, which appears to be spontaneous in certain bodies, such as the sulfides, is the result of their not being able to retain at the ordinary temperature an excess of phosphorescence, against which an antagonistic force, to which I shall refer later on, contends. By cooling them sufficiently, we suppress all emission. They are then analogous to bodies such as fluorite or apatite, which are only phosphorescent above 50° C, and on which time has absolutely no effect.
The action of temperature is therefore much more important than that of time. It is this, and not time, which regulates the emission of phosphorescence.
There can, however, be deduced some useful information from the study of the loss of light under the influence of time if the temperature be kept constant for the whole duration of the phosphorescent emission.
Let us take a screen of insolated calcium sulfide, keep it at 15° C, and examine the curve of the loss of its light. It will fully justify what has been said regarding the part played by temperature.
If this last remains constant, the fall of the curve, representing the loss of phosphorescence as a function of the time, is for the first minute after the insolation almost vertical. It then bends slowly, becomes less and less oblique, and finally quite horizontal. At this moment the body no longer radiates, time no longer influences it, and it keeps intact an invisible provision of luminous energy which it will part with only by various means, or, more particularly, by a rise in its temperature. We will return at length to this last point in another chapter.
The general appearance of the above curve shows, indeed, that things happen as if the reaction exciting the phosphorescence placed itself in equilibrium with an opposing force acting in a converse direction. Immediately after its insolation the screen contains an excess of phosphorescence. Under the influence of the opposing force, this excess is dissipated, rapidly at first, then slowly when that moment approaches when the equilibrium is established between the phosphorescence and the opposing force. When this equilibrium is attained, the reactions which produce the phosphorescence stop entirely. The opposing force being unable to act further on the reaction which generated the phosphorescence, the body will retain its residue of phosphorescence until a rise in temperature again destroys the equilibrium.
I do not know of what this opposing force consists. I can only say that things take place exactly as if it existed. This interpretation has also led me to the discovery of the discovery of the phenomena of invisible phosphorescence studied in a future chapter.
Phosphorescence Produced by Heat
1. Method of Observation ~
Many minerals in nature possess the property of acquiring a bright phosphorescence when brought to a low temperature without first having first been exposed to light. If the heating is sufficiently prolonged, they lose all their provision of phosphorescence, and no longer shine when heated after being allowed to cool. They regain, but in a slight degree, the property of shining by a rise in temperature, when we expose them to the light after having exhausted their phosphorescence by heating.
These facts, which have been known for a long time, represent about all that is to be found in textbooks on physics regarding phosphorescence produced by heat.
It is astonishing that so surprising a phenomenon as the phosphorescence caused by slight heat should not long ago have engaged the attention of physicists. With the theories current it is not apparent in what form a body cold have preserved since its geological formation a provision of luminous energy which we can force it to expend by fringing it to a temperature which may often be less than 100° C.
The experiments now to be mentioned prove that the explanation of the phenomenon is much simpler than that last given. It is not in the form of luminous energy that bodies preserve for ages their aptitude for phosphorescence on a slight rise in temperature. They have simply preserved compounds incapable of combining with each other at an ordinary temperature, but able to do so when it is raised, which is the case with many chemical reactions. Phosphorescence simply arises from these combinations. Bodies in presence of each other which will not combine under a certain temperature, can evidently retain for an indefinite period the aptitude for phosphorescence just as chlorine and hydrogen remain inactive for an indefinite period in darkness, and combine only when we introduce some exciting agent such as light.
If I had not deemed it useless to modify accepted classifications, it is really in the paragraph devoted to phosphorescence by chemical reaction that I should have inserted that which concerns phosphorescence produced by heat.
The methodical study of the action of heat on phosphorescence demands a little apparatus easy to construct. It will serve us not only for study of the action of heat, but likewise for that of the infrared and of invisible phosphorescence, which we shall examine in other chapters.
The method of observation plays an important part in the research into the phenomena of phosphorescence by heat. The processes of observation employed by mineralogists, which consist in placing the bodies to be examined on metal plates or crucibles at a red heat, are quite primitive, and it is because their processes are rude that so many phenomena have escaped them.
The moment the source of heat becomes visible, whether it be produced by a simple spirit lamp, or a gas burner with a blue flame, the eye is dazzled, and all feeble phosphorescence escapes observation.
For the purpose of studying the phosphorescence produced near the regions of the red rays, I simply make use of a large metal box, without a cover, inverted over a small spirit lamp. Its sides should be serrated at the bottom to allow the passage of air, and, in the part over the lamp, an orifice made in which to place a small copper cup one-tenth of a millimeter thick, which receives the body to be heated. By reason of the thinness of its walls this cup can be heated and cooled almost instantaneously. It can be made red-hot with the greatest ease, whenever necessary.
We procure from a dealer in photographic apparatus one of those triple burner lamps with a metal chimney, which are used for lighting magic lanterns. On the upper extremity of the chimney, which is pierced with lateral holes for the passage of air, we place horizontally, in order to stop the light entirely and to have a plane surface to receive the bodies required to be heated, the lid of a biscuit box. The glass or mica plate through which the light of the lamp issues, and which slides in grooves, is replaced by a metal plate which thus masks all the visible rays. As a little light still issues from the lower part of the apparatus, the latter is surrounded by cardboard, which entirely envelopes it.
Figure 22 / 23 ~
Thus constituted, the apparatus is all that is needed for researches where heat alone comes into play. But in many experiments on invisible phosphorescence, we need an abundant source of infrared variations of determinate wavelength. In order to obtain these, it suffices to replace the metal plate in front of the lamp by a strip of ebonite about 0.5 mm thick, fixed between two strips of glass to prevent its shape being altered by the heat. The ebonite may be replaced with advantage by black glass, but this must be of good quality, so that the disc of the sun cannot be seen through it, and yet that it may be transparent to the infrared. This being difficult to procure, I do not dwell on its use.
Under these conditions, the lamp gives the following temperatures: --
(1) With all three burners alight, about 225° C; (2) With two burners, about 130° C; (3) With one burner, about 65° C; (4) Temperature of the vertical side of the metallic chimney of the lamp, about 105° C.; (5) Temperature at 1 cm from the vertical side of the chimney, about 50° C.
In front of the strip of ebonite or black glass, the temperature at 2 mm of their surface is only 30° C., but through these bodies there passes a great quantity of invisible infrared radiations, of which a great part is comprised in the region of the spectrum from 0.3 microns to about 3 microns. It is these which act on phosphorescent substances, and possess the property of passing through opaque bodies, as we shall see in another chapter.
All the metal sides of the lamp also, of course, emit infrared rays, as do all heated bodies, but these radiations, having a wavelength of 5 microns to 10 microns, do not act on phosphorescent bodies.
In this manner is constituted the source of heat and of dark radiations which I have designated by the name of ‘dark lamp’.
The instrument should be placed in an absolutely dark room. It is preferable to make the experiments in the evening, for then the eye is much more sensitive to dim light. If the operations are effected in daylight, one should remain 15 minutes in the dark and let the illumination, whether by magnesium or by the sun, be carried out by an assistant, while the observer remains in the dark. This last precaution is quite indispensable. Many phenomena escaped me at the commencement of my experiments for want of this precaution.
2. The Properties of Bodies Phosphorescent by Heat ~
The list of bodies phosphorescent by heat is very long, though in treatises on mineralogy but a small number a re given: fluorite, fluorspar, topaz, several varieties of calcium phosphate, leucophane, diamond, are noticeable among them. A large number of others must now be added to these different bodies. Among those I have examined I will mention especially the Siberian and Bogotan emeralds, the Auvergne amethyst (but not that of Madagascar), the chlorospinel, the opal, cryolite, scheelite, Wagnerite, phenacite, petalite, castor, pollux, colemanite, talc, baryta, etc.
Most of these compounds are of aqueous origin. Bodies very phosphorescent by light -- that is, the sulfides of the alkaline earths -- only acquire, on the contrary, the property of becoming phosphorescent after having been subjected, during their manufacture, to a high temperature.
Many of the bodies phosphorescent by heat, and especially the Australian opal, leucophane, and the apatite of Estrmadura, together with nearly all the different varieties of fluorite, likewise become phosphorescent by exposure to light; but their phosphorescence, being in that case very slight, is only perceived by the observer after a stay of 15 minutes in the dark.
As the above bodies possess identical properties, and only differ from one another by the intensity of their phosphorescence, its duration, and the temperatures at which it manifests, I shall here confine myself to the examination of some of those which offer the brightest phosphorescence -- such as the apatite of Estremadura and the green fluorite especially.
The apatite of Estremadura, a body easily procured in large quantities, is the one of which the phosphorescence commences at the lowest temperature. Reduced to powder, and put in a tube placed in a sand bath or in water slowly heated in the dark, it begins to shine with a feeble light at 51° C. Heated to 200° C it acquires a bright phosphorescence, which lasts for about an hour, on condition, be it understood, that that temperature is not exceeded. If brought to a red heat its brilliancy will be greater still, but will pass off in less than a minute.
The different varieties of fluorite noticed by me -- and they are very numerous -- behave much like apatite, but are always less luminous.
The temperature at which the fluorites become phosphorescent varies greatly with the different samples. I have examined some 30 of different origins, and have noted that the majority sine at temperatures below 200° C. The varieties which become illuminated at the lowest temperature are those from Morgen (Saone and Loire), from the Pyrenees, and from Schwarzenbergen in Saxony. They commence to shine at about 62° C.
It has been said that the transparent fluorites do not exhibit phosphorescence by heat, This erroneous assertion can only be due to an imperfect method of observation. All the fluorites that I have examined, including some which were colorless and transparent as glass, and which are used in the manufacture of prisms and lenses, are very phosphorescent by heat. I have, moreover, found but one sample of fluorite which was not phosphorescent by heat, i.e., the yellowish cubic variety in little crystals which comes from Herblay.
All fluorites are slightly phosphorescent by light, save the yellow variety above mentioned and a green crystallized fluorite coming from Durham.
When, after the expulsion by heat of all their phosphorescence, the bodies examined above are exposed to the sun and heated anew, they again shine, but much less brightly than the first time.
The property appertaining to several bodies like fluorite, of becoming phosphorescent at one and the same time by heat and by light, has been the origin of an error which for a century runs through all treatises on mineralogy, and has been religiously repeated by various authors, including Becquerel, without their ever having taken the trouble to verify the grounds of it. According to them, certain varieties of the green fluorite called chlorophane shine indefinitely in the dark at a temperature of 30° C -- that is to say, they are luminous in darkness nearly all the summer in our climate and the whole year round in hot countries. This is how Boudant expresses himself with regard to this matter: --
"There are varieties designated under the name of Chlorophane, of which some are phosphorescent at the average temperature of our climate, so that they shine constantly in the dark, while others simply require the temperature of the hand" (Mineralogie, 2nd edition, vol. 1., p. 203).
The most recent and important German treatise on mineralogy, that of Naumann and Zirkel, expresses the same idea: "Many topazes, diamonds, and fluorites become phosphorescent at the temperature of the hand. The green fluorite (chlorophane) often remains brilliant for weeks after being insolated".
Although this fact enunciated by mineralogists seemed theoretically very improbable, I desired, by reason of its consequences, to verify it. I therefore examined innumerable samples of the variety of fluorites called chlorophane from the most important firms of mineralogists in Europe. Not one shone at 30° C., nor at the temperature of the hand.
Evidently this was bound to be so. Suppose, in fact, they could have shone at from 30° to 37° C. Having been many times exposed to this temperature since their geological formation, they must have lost long ago their provision of phosphorescence, unless we class them in the category of radioactive bodies spontaneously and perpetually phosphorescent.
After having discovered that the fact set forth by physicists like Becquerel and mineralogists like Boudant was incorrect, the cause of the error had to be established. Its explanation was very simple.
The fluorites in question acquire, by insolation, a very feeble phosphorescence, which the eye, unless previously rested, does not perceive in the dark. If heated subsequently by holding them in the hand, it happens, as is also the case with all phosphorescent bodies, that their luminosity becomes more vivid. It is then easily seen, and the observer concludes that it is the heat of the hand which has produced this phosphorescence. To be convinced that it is nothing of the kind, we need only leave the sample in the dark for 48 hours. Then, placing it in the hollow of the hand, it is noticed that it no longer shines at all. If it be exposed to the light and then placed in the hand, it will shine at once. Consequently light is plainly the origin of the phenomenon.
The cause of the error, so faithfully reproduced in all treatises of mineralogy, was therefore that operators had left their specimens of fluorite exposed to the light before putting it in their hand. Far from shining all the summer, fluorite actually does not keep this property after insolation longer than ordinary phosphorescent bodies -- that is to say, for a few hours.
What is the origin of the phosphorescence manifested by certain bodies under the influence of heat? Phosphorescent sulfides can retain indefinitely a part of the energy stored up by exposure to light. Might it not then be supposed that the phosphorescence emitted under the influence of heat is due to the fact that the bodies manifesting it have often been manipulated in the light before reaching the hands of the observer? Their phosphorescence would consequently be of recent origin, and analogous to that preserved for years by the sulfides after insolation, which can be made to appear by heating.
In order to solve this question, it was necessary to examine bodies which had not been brought to light since their geological formation.
For this purpose, it sufficed to break up, in absolute darkness, large blocks of apatite and fluorite, to extract fragments from the center of these blocks, and to subject them to the influence of heat. These fragments shone as vividly as those taken from the surface of the blocks. It was therefore not to a residuum derived from the recent origin of light that their phosphorescence was due.
When studying the causes of phosphorescence, we shall see that the light manifested by bodies under the influence of temperature is the result of chemical reaction provoked by heat. In bodies phosphorescent by light, in ordinary temperature, these combinations are easily reformed after being torn apart, and that is why all their phosphorescence is restored to them by exposing them anew to the light when they have become dark. For bodies which only become phosphorescent at a more or less elevated temperature, and only regain very feebly the aptitude for anew phosphorescence by heat after insolation, it is probable that the combinations destroyed by the temperature are only very incompletely reformed by light. We shall see, however, that they may be completely regenerated under the influence of electricity, and that there can be restored to bodies which have lost it, the property of shining anew under the influence of heat as vividly as the first time they were heated.
The aptitude of bodies to again become phosphorescent in the light after having been heated is, moreover, only lost with great difficulty. All those on which I have experimented -- fluotire, leucophane, apatite, etc. -- must be calcined at a red heat for 15 hours before losing it entirely. Ten hours of calcinations were quite insufficient.
How does a protracted rise in temperature act? Why does it take from a body its aptitude for giving phosphorescence?
The first idea which strikes one is that the calcinations destroys certain foreign bodies necessary to phosphorescence.
This hypothesis seems at first sight justified by the change in appearance undergone by a small number of bodies subjected to this calcination. The violet amethyst, when calcined, loses its color entirely, and becomes as transparent as glass. It has therefore evidently lost something which produced the phosphorescence, since this can no longer be restored to it afterwards by the action of light or heat.
But that which is true as regards amethysts and, perhaps, also other bodies, ceases entirely to be so for the great majority of phosphorescent substances. By calcining them for 15 hours, I have caused them to lose entirely their property of becoming again phosphorescent by heat after insolation. But by this operation I eliminated no foreign element. I simply destroyed the aptitude of the bodies present for chemical combination. Proof of this is furnished by the fact that by suitable treatment the aptitude to become phosphorescent by heat as they were before calcinations can be restored to calcined bodies.
To restore to a calcined body its aptitude for phosphorescence, it suffices to pass electric sparks through it for a certain length of time. This property of the electric spark was summarily observed as early as the first years of the last century. As, however, the induction coil was not then known, I thought it might be useful to take up again the study of the phenomenon.
The bodies subjected to the influence of the induction spark were placed in a glass tube closed at its two extremities by corks, through which passed copper rods connected to a strong coil. By approaching and withdrawing these two rods sparks of from 1 to 15 cm could be obtained at will.
The length of the spark has a manifest action. If, for example, calcium carbonate is electrified by a spark of 2 cm, it shines very feebly at 200° C. It shines, on the contrary, very vividly if the spark is 15 cm long.
The duration of the electrification has also a notable influence. If powdered glass is electrified for 5 seconds only, it shines but feebly at 200° C. It shines, on the contrary, vividly at that temperatures if the action of the spark is prolonged for 5 minutes.
In a general way, electricity simply vivifies the phosphorescence of bodies which, without its influence, would have been very weak, but it has never, to my knowledge, communicated it to bodies without any traces of it. In addition, it lowers the degree at which they begin to shine by the action of heat. Cape diamonds, hardly phosphorescent at 200° C., shine brilliantly at this temperature after the passage of electricity through them. Bologna spar, which only shines at 500° C. without electrification, is brilliant at 200° C, so soon as it has been electrified.
There exists indeed a very small number of bodies which present no trace of phosphorescence at 500° C., and give a very slight one at that temperature after electrification. This is, for example, the case with barium bromide. But then it may be supposed that before electrification they manifested a phosphorescence too slight to be visible. I found this conclusion on this general fact -- that electrification only renders more vivid a slight phosphorescence. Even with bodies which possess a bright phosphorescence when they are impure, such as the sulfides of calcium, barium, strontium, and zinc, the electric spark confers on them no trace of aptitude for phosphorescence when they are pure.
The only bodies to which no phosphorescence can be restored by electricity are those to which calcinations really causes a loss. Such is the case with the amethyst, which loses its coloring matter when calcined. To all other bodies phosphorescent by heat, electrification restores the phosphorescence lost by calcinations. I have noted it especially in fluorite, leucophane, and apatite, which after having been calcined for 15 hours, lost the property of regaining phosphorescence by heat after insolation.
In this last case the body heated to 500° C will first emit all it can lose at all lower temperatures, and consequently will no longer be able to shine at less than 500° C. To give it back its phosphorescence, it will have to be heated again to 500° C or more.
This general law of the emission of phosphorescence as a function of the temperature, already pointed out in the last chapter, enables us to unite in one class certain bodies phosphorescent by light and heat, till now separated. We can very easily, in fact, transform bodies phosphorescent by light into bodies phosphorescent by heat. The sulfides of calcium, zinc, and strontium, kept in the dark for days or years after their insolation, are identical with bodies phosphorescent by heat, such as the amethyst, fluorite, leucophane, and apatite. It will suffice also to heat them up to about 70 C to enable us to see them shine.
The analogy may be, however, carried much further. The substances we call phosphorescent by heat only shine at determinate temperatures varying from to 300° C, according to the bodies observed. The luminous sulfides, which shine when insolated at the normal temperature differ from the preceding bodies solely because the temperature at which they cease shining is much lower. Dewar, as has been said, has shown that, in liquid air, insolated bodies acquire no phosphorescence, but that they manifest it so soon as they are allowed to return to the ambient temperature. To bring bodies refrigerated to about -190° C back to the temperature of the ambient air, simply signifies that we heat them to more than 200° C (1). Sulfides which are dark at -190° C and shine at 0° C are analogous to bodies which become phosphorescent by heat at a temperature of +200° C. Both kinds are only phosphorescent when they have been sufficiently heated. A phosphorescent sulfide kept for an indefinite time at -190° C would always remain dark, as would apatite kept indefinitely at a temperature lower than +50° C. The two bodies only differ, I repeat, by the temperature at which the combinations accompanying their phosphorescence take place.
[(1) These are, however, changes much too great. The divers phosphorescent sulfides cease to shine long before -190° C, and return to phosphorescence long before 0 C is reached. The temperature varies, moreover, with the various sulfides.]
When bodies phosphorescent by heat have been brought to the temperature at which their phosphorescence becomes extinguished, they cannot, as has been said, recover by exposure to the light more than a very small part of it. This is probably due to the fact that the chemical reaction which produces the phosphorescence can only be partially regenerated in certain bodies by light, while in others it is totally so, as especially with the phosphorescent sulfides and diamonds. There exists no real barrier between bodies phosphorescent by light and those phosphorescent by heat.
From the above consideration may be deduced the following laws, applicable to all bodies phosphorescent by light or by heat: --
(1) There are no bodies phosphorescent without heat. A body capable of being rendered phosphorescent by light will only manifest its phosphorescence at a certain temperature.
(2) For each phosphorescent body there is a minimum temperature below which exposure to the light cannot produce visible phosphorescence.
(3) To each temperature corresponds a certain emission of phosphorescence which cannot be exceeded.
(4) Bodies which heat renders phosphorescent also become so by light, but only slightly. They differ from those vividly phosphorescent by light only in that in these last the luminous rays entirely regenerate the whole of the destroyed phosphorescence instead of regenerating only a part of it.
Phosphorescence Proceeding From Causes Other Than Light and Heat
(1) Phosphorescence through Impact and by Friction ~
A large number of bodies become phosphorescent by friction or by impact. In the case of some -- apatite, leucophane, diamonds, sugar and uranium -- the impacts or movements may be slight; for others -- such as fluorite and silex -- the friction or impact must be somewhat energetic.
Whether violent or slight, they only generate a very weak rise in temperature, quite insufficient to bring them to incandescence. It cannot therefore be heat which produces the phosphorescence observed in these substances mentioned.
Neither does the shock not the friction determine the preliminary operations necessary to produce a reaction propagated from point to point, as in the decomposition of nitrogen iodide for instance. Phosphorescence by shock or friction does not, in fact, survive the disappearance of its cause, as in the case with that due to light.
The oxygen of the air plays no part in phosphorescence by shock, which is favored, on the contrary, by the total absence of oxygen -- that is to say, by a vacuum. An exhausted tube containing a little mercury becomes luminous as soon as the metal is displaced by a slight movement.
Several bodies which friction renders phosphorescent also become so by the action of light and of heat. There exist, however, a small number such as metallic uranium, which are rendered phosphorescent exclusively by friction.
If, by a very prolonged calcinations, we destroy the property possessed by may bodies -- leucite, apatite, and leucophane -- of phosphorescence under the influence of heat, these substances regain the facility of becoming luminous by friction. We shall notice, on the other hand, that bodies hardly at all phosphorescent by heat, like Cape diamonds, become vividly so by friction.
The result of these experiments seems to be that the reactions which give rise to bodies an aptitude for luminous and caloric phosphorescence differ from those which cause them to shine after friction or a shock. It is probable, however, that it is an action of different causes producing the same effect.
2. Phosphorescence by X-rays, Cathode Rays and High-Frequency Effluves ~
The phenomena of phosphorescence from the above causes are very well known, and shall have very little to add to them.
We know that a large number of bodies, and among them compounds not phosphorescent by other processes, such as rubies, become extremely luminous when exposed to the bombardment of the rarified gases in a Crookes tube. The phosphorescence of those which become brilliant in a strong light, such as diamonds and certain sulfides, is greatly increased. It is sufficient to place them in a Geissler tube subjected to induction sparks. With some calcium sulfide in such a tube, I was able to obtain a phosphorescence of which the brilliancy, surface for surface, reached 7/10 of a candlepower.
The x-rays from a Crookes tube provoke only a very weak phosphorescence, and only in a small number of bodies, contrary to what is generally thought. Even with those phosphorescent in ordinary or in uv light, the illumination is slight/ The x-rays make calcium sulfide luminous to an extremely slight extent, much less than would a simple candle. They also illumine moderately -- that is to say, much less than ordinary daylight -- zinc sulfide, and herein differ entirely from the uv radiations to which it was for a long time sought to assimilate them.
On the fluorescent substances -- that is to say, those of which the phosphorescence does not survive the cause producing it -- the x-rays act, on the contrary, very vividly, therein behaving like uv rays, but more energetically. It was even, as is known, this property which caused their discovery, and rendered them utilizable. Platinocyanide of barium, of which radiographic screens are formed, is not the only salt which becomes strongly luminous under their action. Apatite, leucophane, fluorite, and, above all, Brazilian diamonds are also illuminated, though more feebly.
Many bodies, diamonds, zinc sulfide, etc., which are luminous under the influence of the x-rays, also become so under the action of the salts of radium. Cape diamonds, not phosphorescent by light, become so in presence of radium bromide, even when this salt is enclosed in a thin metal tube.
Sir W. Crookes has remarked that diamonds placed for several months in contact with radium, acquire such a radioactivity that it does not disappear when they are heated to a dark red.
Taken altogether, the preceding phenomena show us that certain bodies become phosphorescent from very different causes -- light, shock, cathode rays, x-rays, etc.; these causes perhaps act, as has been said, by producing reactions, which, if not identical, are at least of the same order.
(3). Phosphorescence by Chemical Reaction ~
For a long time, the only chemical reaction known to produce phosphorescence was the slow oxidation of phosphorus. It is known, by recent investigations, that phosphorescence accompanies a considerable number of reactions.
Most of these, however, are badly defined and result from somewhat complex mixtures. Thus, for instance, those produced by mixing certain organic bodies, such as esculine or various essences with an alcoholic solution of potash, or, again, that obtained by pouring aluminum sulfate or gold chloride into an alkaline solution of pyrogallol (1).
[(1) For producing a rather lively phosphorescence there has been recently mentioned a mixture of: 10 cc 10% solution of pyrogallic acid, 20 cc 40% solution of potassium carbonate, 10 cc 30% solution of formol. The above mixture should become phosphorescent by adding to it 30% of concentrated oxygenated water.]
To arrive at a precise knowledge of the causes of phosphorescence by chemical reaction, it must be produced by reactions far more simple than those preceding -- the oxidation or hydration of well-defined compounds, for instance. This is what I have endeavored to realize.
Phosphorescence by oxidation is exceptional. That of phosphorus is very probably not due to a simple oxidation only, as I have shown elsewhere.
I have obtained other phosphoresences by oxidation, but they border on incandescence as much as on phosphorescence, and constitute perhaps a transition between the two. Such is notably the case with uranium.
Let us take a strip of this metal about 1 mm thick, and place it on a plate heated to about 600° C. It becomes vividly brilliant. Withdraw it with pincers, and it continues to sine for two or three minutes, which would not happen if it were a simple phenomenon of incandescence that was in question. The same operation may be repeated several times, but the duration of the luminosity of the metal becomes shorter each time, and finally -- no doubt when it is entirely oxidized -- heat no longer produces any effect upon it.
The only clearly defined reaction which allowed me to obtain phosphorescence is hydration. The best-defined cases are those of sulfates of quinine and cinchonine. Heated to 150° C, these salts lose part of their water, and after shining for an instant again become completely dark. When they have thus lost all their phosphorescence, they are allowed to cool; they become at once hydrated by contact with the air, again become phosphorescent, and at the same time radioactive. I shall not dwell upon this experiment, the details of which I have given in The Evolution of Matter. By reason of its considerable theoretical importance from the point of view of the causes of radioactivity, it has been the object of important papers abroad. The exactness of my observations has thus been clearly confirmed.
It is possible, in the preceding experiment, to render the employment of heat completely useless. It is sufficient to mix quinine sulfate in a bottle with a little anhydrous phosphoric acid which at once dehydrates it. It is hydrated anew by simply breathing on its surface. Persons not previously informed of the theory of the operation are always much surprised when shown a body which becomes phosphorescent when breathed upon.
Quinine sulfate is, of course, not the only compound which possesses the property of becoming phosphorescent by hydration. I have found a whole series of bodies offering the same phenomenon. Such, especially, are ordinary magnesia, commercial calcium sulfate, and hydrated alumina.
These only differ from quinine sulfate by becoming phosphorescent by dehydration alone, instead of phosphorescing, like the first, both by hydration and by dehydration. Further, as these bodies become dehydrated slowly and with difficulty, they have to be heated to nearly 500 C for the phenomenon to be well marked.
Phosphorescence is very easy to observe with calcium sulfate and especially with ordinary magnesia, which becomes very luminous when dehydrating. This is less apparent with alumina.
If, after heating these compounds for some minutes until the phosphorescence is extinct, they are allowed to cool in the dark and then heated again, they again shine. But the luminosity is much greater if their hydration has been rendered more complete by moistening them with a little water before heating them. As soon as the heat is sufficient to produce dehydration, they become very phosphorescent.
The phosphorescence thus obtained can be repeated indefinitely on the same body by simply moistening it the moment it is dried by the heat. This is not at all the case, as we have seen, with bodies made phosphorescent by a simple rise in temperature. Cooled and then heated again, these last no longer become phosphorescent unless in the meantime they have been exposed to the light.
Salts of radium lose, as I have shown in my earlier work, their phosphorescence when hydrated. The very active ones regain it at once after having been dehydrated by heat, those slightly active, only some days after their dessication.
4. Phosphorescence of Living Beings ~
The phosphorescence of living beings has for a long time been considered as a rather rare phenomenon manifested by a very small number of animals and vegetables.
It has been observed, however, from remote antiquity. Besides the "sea of fire" necessarily known to all navigators by the presence of infusoria, the ancient authors have mentioned the phosphorescence of certain marine animals. Pliny pointed out that the liquid from the pholads renders luminous in the dark the lips and hands of those who eat this mollusk. Fisher relates, in his Manual de Conchologie, that Reaumur "has noted that fragments of these beings remain luminous after their separation from the body, and that when dried, they are able to emit light anew when moistened".
Until recent years the number of phosphorescent animals known was somewhat restricted. No one could have suspected that the depths of the vast oceans, so long inaccessible, where reigned eternal night, were inhabited by innumerable luminous beings. Since suitable instruments have permitted have permitted the study of the inhabitants of seas at depths of several thousand meters, a complete new world has been revealed.
It than became known that the bottom of the sea was covered with veritable forests of phosphorescent polyps; that the smallest as well as the most bulky of the beings inhabiting these dark depths often possessed organs enabling them to light themselves through the abysses in which they live.
The phosphorescent organs of the marine animals reveal very different dispositions. Some are placed in different parts of the body; others in the eye itself, or above it, and it is quite correctly that the latter have been compared to bicycle lamps. Dr. Richard, curator of the collections of the Prince of Monaco, showed me a whole series of phosphorescent marine animals, and notably large fishes, possessing on each side of the body veritable lamps which they can mask at will. All these beings have been the subject of numerous studies, almost exclusively of an anatomical nature.
In addition to the phosphorescent beings which people the depths of the sea, there should be quoted the luminous bacteria which appear on the bodies of fish after their death and before their decomposition. These bacteria can be cultivated very easily by the classic processes. An excellent culture can be made with ordinary water containing about 3% sea salt and 1% asparagine. It is with analogous products that were obtained the bottles sold by the name of "Living Light" at the Paris Exhibition of 1900. I have prepared some similar ones by simply scraping with a knife blade the scales of a herring and introducing the scrapings into bottles of the above liquid. At the end of 24 hours they possess a luminosity which lasts for 3 or 4 days.
Notwithstanding various researches we have not succeeded in determining the chemical bodies which produce this phosphorescence. We only know that it is a phenomenon which can survive the death of the animal. Carus had already seen that the luminous organs of the Italian lamprey, when dried and powdered, regained their lost phosphorescence on simply being moistened.
These phenomena of the phosphorescence of living beings are certainly due to chemical actions requiring the presence of air and water. When these two elements are suppressed it disappears. It has been shown above that for certain well-defined bodies hydration is always accompanied by phosphorescence.
The spectrum of the phosphorescence of living beings seems to present a few variations differing with the animals. It impresses the photographic plate very rapidly. I have been able to reproduce negatives with two minutes of exposure by taking as the source of light, fish which have become phosphorescent after death by the development of luminous bacteria.
The phosphorescence of living beings presents, in its effects, close analogies to the phosphorescence produced by the various bodies we have already studied. It differs form them, in its causes, by being generated neither by light nor by heat. By its effects as well as by its causes, it is near akin to the phosphorescence by chemical reaction examined above.
5. The Phosphorescence of Gases ~
The study of electric discharges in gases demonstrates the part played by electricity in the production of phosphorescence. The various causes studies above regarding this phenomenon, such as light and heat, act perhaps by indirectly exciting electric manifestations capable of producing phosphorescence.
In the phosphorescence of gases the action of electricity is perfectly clear, since it alone can render them luminous. The highest temperatures, even when a whole meter of gas is heated, only gives them a hardly perceptible luminosity; whereas, if they are introduced in a rarified state into a tube through which an electric current is passed, they become greatly luminous. It is even sufficient to place the tube containing the rarified gas in the neighborhood of a high frequency resonator, or, again, to subject it to the action of electric waves. With tubes of helium, the luminosity is brilliant enough for it to be used to detect these last.
The phosphorescence obtained by the electrification of gases is relatively bright, but up to late years there seemed no hope of rendering it intense enough for lighting purposes.
The remarkable discovery of the mercury lamp has shown that lighting by phosphorescence was practicable, and proves once more to what a degree incandescence is independent of phosphorescence.
It will be remembered that this lamp consists of a long glass rube furnished with electrodes -- one of iron, the other of mercury. If, after having made a vacuum in this tube, these two electrodes are connected to a source of electricity, the traces of gas and mercury vapor that it contains become brightly phosphorescent. In this luminosity there is evidently no phenomenon of incandescence, seeing that the temperature is only about 135° C -- that is to say, about much less than that necessary to render a body incandescent. The flame of a simple candle, as we know, has a temperature of about 1700° C.
The spectrum of the phosphorescence of gases, such as that given by the mercury tube, contains very few red and infrared rays, although it has an output much higher than that of the ordinary incandescent light. To obtain this last there must first be produced an enormous quantity of invisible, and therefore useless, caloric rays. Our present process of artificial lighting is barbarous, as has been recognized for sometime. While the ideal method would be to produce only the visible and not the invisible part of the spectrum, a gas flame or the electric arc contains only 1 percent of useful rays, 99 percent of the energy produced manifesting itself in the form of dark heat. With the phosphorescence of the mercury lamp, about 40 percent seems to serve for lighting. The output of the phosphorescent animals, such as the glowworm, is higher still, since nearly the whole of the energy expended is transformed into visible light. Phosphorescence will probably be the artificial light of the future.
It is not for this reason alone necessary to pursue the study of the phosphorescence of gases. I have always suggested that it was too costly for my modest researches, because I am persuaded that its thorough knowledge will open up new vistas on the greatest problems of physics and astronomy.
Phosphorescence by electrical action gives, then, to atoms the same radiating properties as does heat. Intra-atomic energy ought to play some part in the production of this phenomenon, for the gases in the tubes described are strongly ionized -- that is to say, dissociated by the passage of the current.
It is probably to the influence of electric actions analogous to those of which the effects have just been shown that certain stars, composed, as their spectrum shows, of gas, owe their luminous brilliancy. Since gaseous bodies cannot shine by incandescence, as we have seen, other causes than heat must be sought for the light of many stars.
But is some stars are luminous simply by electric actions similar to those studied, it would follow that the stars to which one attributes an enormous heat, may be on the contrary at a temperature relatively low. It is perhaps only at a certain phase of their existence that they shine by incandescence.
The Causes of Phosphorescence
1. Phosphorescence as a Manifestation of Intra-Atomic Energy ~
Phosphorescence represents the transformation into light of various energies of which only a few are known. In the case of gases we have seen that electric energy is the sole possible cause of the phosphorescence. In that due to the action of light and of heat, and of chemical reactions, other energies intervene, the mode of action of which is still undetermined.
Since very different causes -- light, shock or impact, and x-rays -- may produce phosphorescence in the same substance, there is ground for believing that these very different excitants act by provoking the manifestation of reactions, which, if not identical, are at least of the same order. The old theory which considered phosphorescent bodies as simply restoring the light absorbed, as a sponge restores the water it has imbibed, is no longer tenable at the present day.
The phenomenon of phosphorescence seems linked with that of the dissociation of matter, and with the liberation of energies which accompany this dissociation. The link is evident as regard the phosphorescence of gases, since the latter only become phosphorescent under the influence of electrical actions, which are always accompanied by ionization -- that is to say, by the disintegration of their atoms.
As regards other forms of phosphorescence, the connection with dissociation is far less evident. It appears, however, at least probable in the case of phosphorescence by light, when we remember that light energetically dissociates matter, and that it is just the radiations producing this dissociation which are the most apt to produce phosphorescence.
The tendency to consider phosphorescence as one of the consequences of the dissociation of atoms is beginning to take shape among physicists. De Heen and, after him, Lenard, have recently arrived at the conclusion that light acts by provoking the emission of negative ions issuing from the atom, and capable of returning to it by oscillation.
To these movement of the elements of the dissociated atom should be due the radiation in the ether producing phosphorescence. Its light, of the same nature as that of incandescent bodies, differs from it by the absence of any elevation of temperature, which amply justifies the name of cold light sometimes given to it. It is possible that the rays of light, electricity, and the different causes of phosphorescence bring the intra-atomic energy immediately into play without its having to first pass through molecular movements, as in the case of heat.
It is not strictly proven, moreover, that cold light is not in reality quite as hot as that generated by incandescence. If phosphorescence is a very superficial atomic phenomenon, it may be that the elevated temperature accompanying it is not appreciable, Crookes has observed that by exposing diamonds to the cathode bombardment, their surface is transformed into graphite -- which implies, according to Moissan, a temperature of 3600 C -- and yet the deeper strata of the diamond experience no notable elevation of temperature. The tube in which it is enclosed is very little heated. The same author has likewise observed that the surface of silver plate can be thus brought to a red heat, while the temperature of the whole mass of metal is hardly raised, notwithstanding the high conductivity of silver for heat. I must remark, however, that after rendering phosphorescent large masses of certain sulfides, spread out so as to have only a thickness of some hundredths of a millimeter, and afterwards collecting the mass in a flask, its temperature was not appreciably raised.
The above consideration on the intra-atomic origins of phosphorescence only constitute the suggestion of an explanation; the complete solution of the problem is still far off.
It is of importance to first thoroughly determine the reactions producing phosphorescence. They are of a special order, for which the laws of the early chemistry are of no service. How, in fact, could be have conceived that reactions bringing into play significant quantities of energy as, for example, the very slight hydration of a salt of quinine, could act on so stable a structure as an atom, and at the same time generate both radioactivity and phosphorescence.
Without being able to entirely elucidate such phenomena, one can at least grasp its possibility by bearing in mind the notion, repeated referred to in the book, that the atom, notwithstanding its stability, may become unstable when we bring to bear upon it a suitable reagent. It then behaves somewhat like a tuning fork, which the most intense noise is powerless to shake, while a slight sound of suitable period will cause it to vibrate. A thin pencil of uv light will thus dissociate without difficulty the atoms of a block of steel capable of withstanding the most violent shocks. When a salt of quinine becomes very phosphorescent and radioactive solely by the addition of a thousandth part of its weight in water-vapor, we have simply -- without, however, clearly knowing how -- excited reactions giving to the atoms of this body the instability which precedes dissociation.
2. Special Character of the Chemical Reactions Giving to Bodies the Aptitude for Phosphorescence ~
What has been said will enable us to catch a glimpse of the reason why bodies, never phosphorescent under the influence of light when pure, acquire this property when we add to them a few hundred-thousandth parts of their weight -- that is to say, almost imponderable traces -- of foreign bodies.
The sulfides of the alkaline earths and natural minerals are never phosphorescent in a state of purity. Certain bodies considered as very pure, such as the fluorite used to make lenses, crystallized apatite, etc., are, however, phosphorescent, but their pure state is only apparent. They always contain traces of foreign bodies. So does the diamond, as chemical analysis of it shows.
It is the special combinations due to the presence of these foreign bodies which give to certain chemical compounds the aptitude for phosphorescence. Once formed, these combinations present some curious characteristics. Very movable, as is proved by the rapidity with which the different rays of light cause phosphorescence to appear or disappear, they nevertheless withstand energetic causes of destruction, since it takes 15 hours of calcinations at red heat to deprive certain substances of their aptitude for phosphorescence. This calcinations in no wise acts by eliminating anything, since the regeneration of the combination and the aptitude for phosphorescence which ensues are obtained by passing induction sparks through the calcined bodies. Heat has not therefore eliminated any foreign body, but has simply destroyed the chemical combinations capable of being accompanied by phosphorescence, which reform under the influence of the electric spark.
The foreign bodies capable of causing phosphorescence must always be present in infinitesimal proportions. Different substances may be substituted for one another to produce effects, if not identical, at all events analogous. M. Mourelo has shown that, to give strontium sulfide the property of becoming phosphorescent by heat, it has only to be calcined with a ten-thousandth part of a salt of manganese or of bismuth. Similar observations have been made with regard to the other sulfides.
The early experiments of Becquerel prove that the slightest variation in the composition of the bodies in the mixture have an influence on their phosphorescence. Marble, chalk, Iceland spar, bodies chemically identical, since they are composed of calcium carbonate, when dissolved in nitric acid give calcium carbonates which we might suppose to be identical. They are not, however; since, by calcining with sulfur the calcium carbonates of various sources we obtain sulfides of calcium of which the phosphorescence may be yellow, green, or violet. The carbonates of lime employed have thus retained infinitesimal traces of foreign bodies varying with their origin.
One of the most singular characteristics of the chemical combinations capable of giving to bodies the aptitude for phosphorescence by light is, as is said above, an extreme mobility -- that is to say, the faculty of being formed, and of being destroyed indefinitely, in an almost simultaneous fashion. Certain radiations produce phosphorescence in zinc sulfide in one-tenth of a second, and others destroy it in the same space of time. Different animals, such as the glow-worm, likewise possess this property of causing their phosphorescence to appear and disappear instantaneously.
This notion of chemical reaction capable of being produced in the heart of perfectly solid bodies, such as diamonds, fluorite, and sulfides of alkaline earths, is evidently still outside the range of classic ideas.
These last hardly admit, according to the adage of the early chemists, that bodies can act on one another save in a state of solution; nor do they admit combination in proportions neither simple nor definite. Chemical science, moreover, has no knowledge of combinations which can almost instantaneously be made, unmade, and remade indefinitely under such slight influences as a ray of light.
Of what do these combinations consist where one of the elements is in an infinitely small proportion relatively to the other?
We know by all that has been said that the combinations which render phosphorescence possible realize the following conditions: (1) The bodies, the addition of which produces combinations accompanied by phosphorescence when excited by light or by heat, ought to be added in very small proportions; (2) the combinations formed are mobile and capable of being regenerated, since they are destroyed and remade in a small fraction of a second; (3) these combinations, so rapidly destroyed and so rapidly regenerated, are intimately connected with the action of the temperatures, which, when it is raised, destroys them very quickly, and very slowly when it is sufficiently low. The phosphorescent body emits for months invisible radiations. The return of the combination to its primitive state is completely stopped at a still lower temperature, and the body preserves it phosphorescence indefinitely until its temperature is raised.
Apart from phosphorescent bodies we do not know any chemical combinations able to realize these various conditions, and simple mixtures certainly do not do so. We are therefore obliged to admit that we have to do with an utterly unknown order of chemical reactions. They will probably remain unknown for a long time, because their extreme instability and the ease with which they are destroyed and regenerated protect them against all processes of chemical analysis in present use. To determine the bodies present is of no use, since it is the combinations formed by them which we want to grasp.
The problem is further complicated, because the foreign bodies, the combinations whereof induces the aptitude for phosphorescence, seem only to act by giving instability to the atom so as to allow it to liberate the energies it contains.
We are hardly in reality beginning to suspect the causes of phosphorescence, but that which we catch a glimpse of enables us to feel by anticipation that it will constitute one of the most important chapters of chemistry, and will surely be linked with the history, as yet hardly outlined, of the dissociation of matter.
Black Light
Chapter I
Invisible Phosphorescence
1. The Divisions of Black Light ~
The appearance in 1896 of the work of Roentgen on the x-rays determined me to publish immediately in order to settle the order of dates, a note on some particular radiations capable of passing through bodies, which I had been studying for two years and which were attached to no known phenomena. I called them by the name of Black Light by reason of their sometimes acting like light while remaining invisible.
These radiations, which I had not had time to separate, at that period, were composed of three very distinct elements: (1) Radioactive particles of the family of cathode rays; (2) radiations of great wavelength; (3) radiations due to invisible phosphorescence. I have already set forth in The Evolution of Matter how I managed to distinguish these different elements in their order.
The first of the three categories of radiations enumerated being identical with the cathode rays and radioactive emissions, it would be useless now to give them a special name. I shall therefore only designate by the name of Black Light: (1) the invisible radiations, totally unknown before my researches, emitted by certain phosphorescent bodies; and (2) the radiations of great wavelength, belonging to the infrared part of the spectrum. This region has been known for a long time, but the majority of its properties have been ignored. It was not suspected before my researches that these radiations passed through a great number of bodies, allowed instantaneous photography in the dark, and possessed very special physiological actions.
The first category of radiations enumerated above have the same composition as ordinary light, and only differ from it by their invisibility. Designating them, together with those of the infrared, under the term Black Light, completes the continuous scale of invisible radiations.
Black Light, then, comprises: (1) the invisible light emitted by certain phosphorescent bodies; (2) the invisible infrared light which in the solar spectrum goes up to 5 microns, and possesses consequently an extent ten times superior to that of the visible spectrum.
I will now study the division of the Black Light constituted by invisible phosphorescence.
2. History of Invisible Phosphorescence ~
When I published in 1899 and 1900 the experiments to the description of which this chapter is devoted, they seemed so astonishing to physicists that they preferred not to believe them. Yet the verification of the most fundamental of them was extremely easy, and demanded no other expense than 50 centimes in money and a few minutes of time. I now know, however, that several repeated them, but, astonished at their success, preferred not to speak of results which were evidently of no account, since official science had not consecrated them. Even today it is with great timidity that they are noticed in a few treatises on physics. Thus, for example, a distinguished scholar, M. Gariel, Prof. of Physics at the Faculte de Medicine de Paris, after having given a summary of them, says: "These effects are almost extraordinary. There is no occasion, however, to put them on one side, for the phenomena relating to radiations are certainly not yet all known" (Physique Biologique, vol. 11, p. 261). This quotation at least shows that if the discovery of new facts is sometimes difficult, it is still more difficult to get them admitted.
The invisible phosphorescence which I discovered is characterized by the following phenomena: (1) A phosphorescent body exposed to the light preserves for a period of about 18 months the property of emitting invisible radiations capable of refraction and polarization, and of impressing photographic plates. The spectrum of these radiations, which is analogous to that of light, only differs from it by its invisibility. (2) At the end of these 18 months, the body has no longer any appreciable radiation, but preserves indefinitely a residuum which can be made visible by projecting on its surface dark infrared radiations.
These phenomena were completely unknown, and nothing warranted their being foreseen. No doubt it was known from all time that many bodies are phosphorescent by heat, and consequently have preserved since their geological formation an aptitude for phosphorescence which appears as soon as they are heated. But as these bodies radiate absolutely nothing in the dark before being heated, they give birth to no invisible phosphorescence. That which they manifest by heat is a very visible phosphorescence.
Notwithstanding their long researches on phosphorescence, E. and H. Becquerel were ignorant of the phenomenon of invisible phosphorescence. Never did they suspect that bodies on which light had fallen for a minute could spontaneously emit in the dark invisible radiations during many long months. While knowing, as Canton had observed, that a phosphorescent sulfide, heated some time after insolation, again became slightly luminous for an instant, E. Becquerel supposed that the spontaneous emission of radiations quickly ceased, and that heat was necessary to expel the slight phosphorescent residue preserved indeed, but, according to him, never for long. This is, moreover, what he says in his book on Light (vol. 1, pp. 52 and 59): "When these substances are exposed to sunlight and placed in profound darkness for a short time -- say, for 3 or 4 days -- they almost entirely lose the faculty of shining immediately by a rise in temperature. Thus, the modification acquired by the action of radiation is only preserved in part and for a certain time in phosphorescent bodies, and then disappears".
The spontaneous invisible radiation produced without any intervention of temperature had thus escaped the notice of this eminent physicist. It is not at all true that "the modification acquired by the action of radiation is only preserved for a certain time, and then finally disappears". We shall see that a part of the modifications impressed by light on phosphorescent bodies is preserved indefinitely and even after they have ceased to emit any invisible phosphorescence, which, however, only happens at the end of about 18 months.
There are two forms of invisible phosphorescence: (1) the one following visible phosphorescence; (2) the one preceding it. They can both be easily transformed into visible light.
3. Properties of Invisible Phosphorescence ~
The invisible phosphorescence which follows the visible constitutes one of the least known and most curious forms of light. It would have been difficult, before my experiments, to foresee that a body exposed for a minute to the sun and then kept in darkness for 18 months preserve the property of emitting, without cessation, radiations identical with light and only differing from it by an absolute invisibility.
The majority of bodies struck by light preserve sometimes for a very long time the property of emitting dark radiations capable of impressing photographic plates. But it is with those capable of first acquiring phosphorescence that the phenomenon can be best studied.
First, here are the experiments by which I determined the properties of the light thus emitted.
(1) Duration of the emission and variation in the intensity of the rays emitted as a function of the time ~ Calcium sulfide in powder placed between two strips of glass is exposed to the light for a few seconds, and then transferred to the drawer of a cupboard placed in a dark room in which no light has penetrated during the course of the experiments -- that is to say, for several years. At the end of 24 hours the screen has become entirely dark. Without taking it from the dark room, it is placed on a photographic negative, under which is placed a gelatino-bromide plate. Still keeping in darkness the system thus constituted, the following is observed:
Three days after the insolation, a very vigorous image of the negative is obtained in two hours. At the end of 15 days the exposure has to be for 12 hours; after 25 days, 30 hours; after 6 months, 40 days. At the end of 18 months traces of the image can still be obtained after an exposure of 60days.
The above proves that the residual charge given by two seconds exposure to the sun has taken 18 months to gradually disappear.
(2) Propagation in a straight line, and refraction ~ The propagation in a straight line and the refraction of the dark light which remains are shown by the following experiment:
A statue coated with calcium sulfide dissolved in copal varnish is exposed for a few seconds to the light. Three or four days after it has become entirely dark, it is placed in a photographic dark room in a cellar into which the daylight has never entered. The focusing has been arranged beforehand. By using a portrait camera with large aperture, we obtain, by exposures varying from 8 to 15 days, images as perfect as those taken in daylight. The shadows vary at the will of the operator, since they depend solely on the position given to the stature during the insolation.
Figure 24 / 25 ~
Figure 26 ~

(3) Polarization ~ The double refraction and consequent polarization of this dark light are shown by the following experiment: A strip of Iceland spar is introduced into the optic system of the camera previously used, and the statue is replaced by two glass tubes in the form of a cross, filled with sulfide and fixed in a place settled beforehand so as to obtain a good focus. By working as before, a few days after the sulfide has ceased to emit light, we see on one of the exes of the cross two partially superposed images, of which the intensity is one half less than that of the part not duplicated -- which is in conformity with the theory (Figure 26).
This experiment proves, at the same time, the emission of invisible radiations, their propagation in a straight line, their refraction, and their aptitude for polarization.
(4) Composition of the Rays emitted ~ The perfect sharpness of the images obtained in the preceding experiments already proves that the index of refraction of the lenses for dark rays is the same as for visible light. Had it been otherwise, the preliminary focusing by ordinary light would not have been exact for rays of different wavelength, especially with the portrait camera employed, of which the focal depth is almost nil. But that is only an indication. To ascertain the composition of the active rays, it would have been necessary to disperse them by a prism and to photograph them. This experiment was not realizable on account of the necessity of employing, in order to obtain fairly clear photographs of the prismatic spectra, a very fine slit which absorbs nearly the whole of the light. The difficulty has been obviated by employing an artificial spectrum, composed of bands of colored glass fixed on a strip of colorless crown glass. This spectrum was first exposed to ordinary light over a sensitive plate, and the image thus obtained, after development, was compared with successive images obtained by interposing the artificial spectrum between the dark screen of sulfide and the sensitive plate. The images were identical in the two cases -- that is to say, nil from the red to the green, and very intense under the blue glass.
From these experiments we may conclude: -- (1) That there is identity of composition between the visible solar light and the dark light emitted by bodies exposed to luminous radiations for a moment. The second only differs from the first by its invisibility, which results from the small amplitude of the waves emitted. (2) That this residual and invisible light lasts for a long time.
4. Persistence of the Aptitude for Phosphorescence after Cessation of the Spontaneous Radiation -- Transformation of Invisible Phosphorescence into Visible ~
We have just seen that certain phosphorescent bodies can for 18 months emit invisible radiations, but that a time arrives when the emission ceases entirely. We shall now see that those dark bodies, which after a period of radiation so long seem to have lost all their energy, preserve indefinitely a certain provision of residual phosphorescence. We shall render it visible at any moment -- after a 10 years’ stay in the dark, for example -- by letting certain invisible radiations fall on the surface of these bodies. They then become brightly phosphorescent enough to be photographed in a few minutes in the dark room.
I noted this fact for the first time with calcium sulfide screens which had been used for my former experiments, and which no longer gave photographic impressions at the end of 18 months, even after 6 weeks’ exposure. They were then left in the dark, where they still are after more than 10 years.
Let us put one of those screens in the dark into a plate-carrier, and cover over the glass with a sheet of black paper or a plate of ebonite, bodies opaque to ordinary light, but very transparent, as has been shown, to dark radiations of great wavelength. Let us expose this plate-carrier for 20 or 25 seconds to a paraffin lamp, and then take it into the dark to open and examine it. We shall find that, under the influence of the invisible radiations, the screen, which has been dark for so many years, has become luminous. Its phosphorescence is sufficient to give a photographic impression by contact in two or three minutes, while before it did not give one after 6 weeks’ exposure.
The phosphorescence thus produced disappears rapidly, but the experiment can be repeated with the same screen more than 50 times at certain intervals -- that is to say, for an indefinite number of years. There naturally arrives a moment when, the phosphorescent residue being exhausted by these successive experiments, the dark rays will produce no effect unless the screen is subjected to a fresh insolation. It is therefore really a case of a limited residual charge, which does not renew itself spontaneously, but which we can keep indefinitely before expending it.
A large number of bodies possess in this way the property of acquiring a provision of residual light, a part of which disappears spontaneously, while the other part is preserved indefinitely. With some of them, such as calcium and strontium sulfide, the invisible residual light may become visible simply under the influence of dark radiations of great wavelength, even when the screen exposed to these radiations is maintained at a very low temperature -- for example, between two glass troughs a centimeter thick, full of ice. Heat therefore cannot be given as the cause of the phenomenon. It is important to note this, for heat by itself can produce the same effect if we raise the temperature to 30° C. The action produced by heat is, moreover, very different from that generated by the dark radiation, as will presently be shown.
The above experiments succeed very well with several phosphorescent sulfides, especially that of calcium, but not at all with zinc sulfide. The reason of this, to which I shall return elsewhere, is that the radiations of great wavelength, capable of destroying the phosphorescence of this sulfide, are totally incapable of exciting it. Zinc sulfide, like other sulfides, retain a residual charge indefinitely, but this residual charge can only be expelled by a temperature approaching 100° C, and not at all by the dark infrared rays.
Many other bodies -- the diamond, for instance -- exist which can indefinitely retain a residual phosphorescence, which may be rendered visible by heat, and not by the infrared rays. A Brazilian diamond exposed to the sun acquired a phosphorescence which vanishes rapidly; but it retains an invisible phosphorescence which can be rendered visible after a few years by heating it to about 200° C.
Instead of effecting the preceding experiments with phosphorescent screens requiring some little preparation, they can be realized more simply by placing the calcium sulfide in a tube placed, after insolation, in a box closed by a sheet of ebonite, or, better still, by a sheet of glass coated with the so-called Japanese varnish, in a layer thick enough for the sun to be invisible through it (1). At the end of 24 hours, the sulfide will no longer shine, but when kept in the dark it will retain indefinitely the faculty of becoming luminous when the invisible radiation above referred to fall on its surface.
[(1) To get the layer of the required thickness, put a little raised border of cardboard around the glass. The layer may be of any thickness. I have observed that a thickness of one cm is easily traversed by the infrared radiations. The only inconvenience of over-thick layers is that they take more than a week to dry. However, a layer of 1 mm is sufficient to give an absolute opacity to the eye, which can be tested by holding it up between the latter and the sun.]
To prove this, we have only to expose for one minute the box containing the calcium sulfide tube to a paraffin lamp. On afterwards opening it in the dark, it will be seen that the tube has become luminous. It is this very easy experiment that I alluded to at the beginning of this chapter.
To render the preceding experiments still more demonstrative, I place at the bottom of a large cardboard box, closed as indicated above, some bas-reliefs coated with a layer of calcium sulfide, dissolved in bronzing varnish. This box is left in the dark. If, at some later period, it is exposed, still closed, to a lamp for two minutes, and then opened in a dark room, the statues will be luminous. The operation may be carried out several weeks after insolation.
Up till now we have always used, to excite the extinguished phosphorescence, visible radiations passing through an opaque screen, which renders them invisible. But the experiment in this form allows it to be supposed that visible light has been able to pass through a chink in the box and to illumine the sulfide. We will now suppress all visible sources of light, place the observer in complete darkness, and in this darkness cause gradually to appear before his eyes a luminous statue which no ray of visible light has touched.
Figure 27 ~

Though very striking, this experiment is most simple, and easily deduced from what is said above. The reader who has thoroughly understood my explanations sees at once that, if we shut up the lamp in an opaque box instead of the statue, the result will be the same. The operation is as follows: --
In a dark room, or, if you do not have one, at night, the dark lamp described above, which allows no visible light to pass, is placed on a table. In front of it is a statuette coated with calcium sulfide which has been left for several days in the dark, and consequently presents no trace of phosphorescence. All being thus prepared, the observer sees, at the end of one or two minutes, the statue light up and come forth from the darkness.
The experiment is a very curious one, and has always vividly impressed the spectator. It is, in fact, very strange to see the dark radiations of the lamp, added to the dark radiations of the sulfide, produce visible light. It is almost the converse of the celebrated interference experiment of Fresnel, in which light added to light produced darkness. In my experiment, it is darkness added to darkness which gives birth to light.
The light thus obtained is not very vivid. It is enough so, however, to enable a photograph of the statue to be takes with an exposure of 40 minutes, with a portrait camera suitably placed beforehand. The dark lamp, of course, is kept near the statue all the time the operation lasts, for if it were taken away, the phosphorescence would extinguish itself in less than a minute. Figure 28 gives the reproduction of statuettes obtained by these means.
Figure 28 ~

The above proves that the residual luminosity stored up by certain bodies is formed of a transitory elements and a permanent one, both being capable of transformation into visible light. But while the transitory element disappears spontaneously by radiation in a greater or less space of time, the permanent element does not radiate spontaneously, but persists indefinitely until artificially expelled, either by calcinations or, without any rise in the temperature, by exposing the body to dark radiations of a certain wavelength.
5. Invisible Phosphorescence Preceding the Visible ~
The invisible phosphorescence which, as we have just seem, follows the visible, may precede it. This is proved by the following experiments.
Let us take a screen made of a phosphorescent body which is slowly impressed by light, such as strontium sulfide, and deprive it, by heat, of all residual phosphorescence. Let us then place it in the plate-carrier of a camera obscura having a diaphragm timed to 1/5 second, point the apparatus towards the sky, and uncover the diaphragm so that the plate is exposed to light for that period. On opening the frame afterwards in the dark, we notice that the sulfide is not luminous, but it will suffice to place it on a plate heated to 200° C for illumination to occur. Its short exposure to the light had thus given it an invisible phosphorescence.
The experiment may be effected more simply with other bodies -- Iceland spar, for example. This compound acquires a very slight visible phosphorescence by heat and none by light, but if insolated and then heated to 200° C, it shines with brilliance for some minutes, which proves that the light communicates to it a certain quantity if invisible phosphorescence. This series of operations can be repeated indefinitely -- that is to say, we can restore to the spar the same luminosity by heat after having insolated it.
6. Comparative Effects on Phosphorescence of the Infrared Radiations and of Heat ~
The first observers, having studied the action of the various parts of the spectrum on bodies capable of phosphorescence, very soon noted that the radiations going from the blue to the ultraviolet produced phosphorescence, and that those from the green to far on in the infrared extinguished it.
H. Becquerel believed that he had found the explanation of this destructive action of the infrared, by saying that it acted simply as a source of heat, and consequently obliged the body to expend very quickly its provision of phosphorescence. This explanation has, from that time, been repeated in all standard works on physics.
Yet it only required very simple experiments to show that it was founded on a mere appearance, which, moreover, does not exist with all phosphorescent bodies. The following experiments enable the parts played by the infrared rays and heat to be clearly differentiated.
Fasten on the same cardboard two small screens, one of calcium sulfide, the other of zinc sulfide, and expose them, after insolation, to the infrared radiations of our dark lamp. The calcium sulfide will become more brilliant, but the zinc sulfide becomes extinguished directly without any increase in light.
But this is only a first indication. I shall show that heat and infrared exercise on phosphorescence two very different actions. They may be added together, whence the error of interpretation pointed out above, but one of them may also act in the contrary way to the other.
We will first examine the case in which these two effects, the specific action of certain radiations and of heat, seem identical.
Let us take a screen of calcium sulfide, which has been insolated for 15 minutes, and expose it to the action of our dark lamp, either in front of the metallic chimney, or of the part closed by ebonite which allows the infrared to pass. In both cases the phosphorescence will first be made more active and then extinguished. Only the action of the heat is slower than that of the infrared, because it only acts when the surface of the screen has had time to get heated.
The same results occur if we employ a screen which has been insolated for a few days and is consequently dark. It will shine in front of all parts of the black lamp -- that is to say, by the action of heat as well as by that of the infrared.
By confining ourselves to these experiments we should arrive at the conclusion, as did the earlier observers, that the infrared rays acted by heating the phosphorescent screen.
To show the inaccuracy of this interpretation, we will repeat the preceding experiment, but interpose between the screen of sulfide and the lamp a strip of glass to prevent the heating of the phosphorescent matter. The effects will then be very different.
In front of the ebonite, a region low in temperature, but which allows many of the infrared rays to pass, there will still be illumination. In front of the chimney of the lamp, a region fairly warm and acting as a heated body, there is no longer any illumination. The strip of glass interposed, which prevents the action of heat, prevents likewise the phosphorescence. It is therefore evident that the action of heat and of radiations of great wavelength are very different.
It evidently might be objected to the above that if the infrared possesses the specific action I ascribe to it apart from the caloric power, the chimney of the lamp of which the action is prevented by the interposition of a glass should have an effect, since it produces infrared rays to the same extent as heated bodies. But as the heat of the walls of this chimney hardly exceed 100° C., the waves emitted have a length not less than 5 to 6 microns, and these have no specific effect on phosphorescence. They act solely by the heat they are able to produce after a certain lapse of time, which is why the interposition of a strip of glass suppresses all such action. Through ebonite, blackened glass, etc., there are emitted radiations of about 0.8 up to 3 microns, which possess a specific action independent of the caloric effect which they must produce in the course of time, and that is the reason they instantaneously act on phosphorescence.
To render the above theory still more convincing, I will now show that the infrared can produce on the two halves of the same screen contrary effects, the one by its specifics, the other by its caloric action.
For the calcium sulfide screen, let us substitute one of zinc sulfide with green phosphorescence. Let us insolate it by daylight, place one half in front of the metallic chimney of the lamp (that is to say, in front of a source of heat), and the other half in front of the ebonite which masks the flame and allows the infrared radiations to pass. On the two halves of the screen, the effects will be diametrically opposite. In front of the ebonite, the screen will become at once extinguished, without any preliminary increase of the phosphorescence. In front of the metal chimney, its phosphorescence will be, on the contrary, markedly increased.
If, instead of having been insolated before exposure to the lamp, the zinc sulfide screen has remained for some time in a dark room, so that it no longer manifests any visible phosphorescence, the difference of action between heat and the infrared radiations will still continue to show itself. The screen will again become phosphorescent before the heated metal wall, and will remain dark before the ebonite, since the rays cannot destroy its visible phosphorescence owing to its being already extinguished (1).
[(1) In all these experiments in which luminous fields of unequal intensity are compared, it is well to put on the screens (on the side facing the lamp) a narrow sheet of tin, which preserves the intensity which they would have had without having been exposed to any radiation.]
These experiments put in evidence the fundamental differences existing between the effect of heat and the specific action of certain radiations.
The above demonstration may be completed by enclosing the phosphorescent screen between two troughs of frozen water 1 cm thick, before placing it in front of the infrared radiation of the lamp. Though its surface cannot, under these conditions, become heated, there will be observed in front of the ebonite effects of illumination in the case of the calcium sulfide, and extinction in the case of the zinc sulfide, as already described.
It is therefore evident that infrared radiations may have specific actions quite independent of those produced by raising the temperature of the bodies absorbing them.
Our experiments having proved that heat and infrared radiations are able in some cases to produce similar effects on certain phosphorescent sulfides -- especially that of calcium -- it became of interest to inquire to what temperature these bodies should be brought in order to obtain by heating them effects identical with those obtained at a low temperature with infrared radiations. Nothing can be easier, since it suffices to determine the temperature at which calcium sulfide, after being insolated for a few days, and thereby darkened, can regain its luminosity. This is effected by placing it in tubes introduced into a receptacle full of water containing in it a thermometer, and heating it gradually in darkness. We thus become aware that calcium sulfide which has been insolated for 8 days only commences to shine at 60° C, and not before 55 seconds, the time necessary to heat it. The same tube of sulfide becomes, on the contrary, instantaneously luminous when exposed at a low temperature to the action of infrared radiations.
One may also wish to discover how much phosphorescence the infrared is capable if taking away from luminous sulfides, and thus fix its caloric equivalent. This is effected by introducing into a photographic plate-carrier, with a thin shutter of ebonite, a screen of calcium sulfide, and exposing it to the sun for several hours. It is then observed that, to restore its phosphorescence, it must be raised to a temperature slightly above 100° C. The great radiations acting at the ordinary temperature have therefore taken from a phosphorescent body all the residual light it might lose by heating it to about 100° C.
7. Radiations of the Metals and of Different Non-Phosphorescent Bodies ~
With invisible phosphorescence there are apparently -- but only apparently -- connected certain impressions obtained by bodies placed in contact, and in the dark, with a sensitive photographic plate. A gelatino-bromide plate is placed in a dark slide under a strip of metal -- zinc, aluminum, or platinum. By interposing between the strip and the metal a cross made of various substances, we generally obtain, after a few hours exposure to the sun or to a strong paraffin lamp, an image of the object interposed, even when it is separated from the metal by a thin plate of mica.
This experiment, and others of the same order which in former years caused me to waste much time, succeed very irregularly, and at the end of a few days the metal no longer gives any image.
Figure 29 ~

These effects are in no way connected with phosphorescence, but with the radioactivity of the metal, and this is the reason that they can be increased by slight heat. The absence of action on the metal -- its fatigue, if we may call it so -- is likewise observed, as I have shown, in the discharge of the electroscope. It is owing to the fact of the metal having expelled, under the influence of slight heat, a provision of radioactive substance, which cannot be regenerated without a long rest, that it becomes inactive.
These radioactive actions, which I confused at the beginning of my experiments with those of the infrared and invisible phosphorescence, were the cause of many researches before I could distinguish them. From time to time different observers come across my early experiments, and, as Dr Russell, Dr Kahlbaum, and Prof Melander have done, observe afresh such impressions. The causes of these being determined, these experiments no longer present any great interest, and that is why I do not dwell on them.
It is not metals alone, as I have already said, which may give such impressions, but wood and animal tissues also produce them [N-rays? -- Ed.]. They are made more active by slight heat; but it is evident that with these different substances certain chemical reactions may also come into play.
The Infrared Rays and Photography Through Opaque Bodies
1. Visibility through Opaque Bodies ~
We have seen that the greater part of the solar spectrum is formed of invisible rays situated in the region of the infrared, and extending for sunlight up to 5 microns, according to the researches of Langley. The spectrum of flames is much longer still, and extends up to 60 microns.
This very important region has hardly been studied hitherto, except from the point of view of its caloric properties. Having discovered that zinc sulfide with green phosphorescence was nearly as sensitive to a part of these radiations as was gelatino-bromide of silver to visible light, I was able to study their properties, especially the one of passing through a great number of opaque bodies. They allow us to see and to photograph through these last. These phenomena will be dealt with here, and the other actions of the infrared will be examined in a future chapter.
For the eye to discern an object placed behind a body supposed to be opaque -- a wooden plank or a piece of black paper, for example -- it is evidently necessary that the rays should first pass through it. It is afterwards necessary that the eye should be rendered sensitive to these rays.
The first of these conditions having always been considered as impossible, no one could dream of realizing the second.
The discovery of x-rays proved that opaque bodies can, indeed, be traversed by certain radiations, but the properties of these radiations, created artificially by our instruments, could not modify the old ideas as to the opacity of bodies for light. The ancient fable of the lynx, whose sparkling eyes could see through walls, seemed destined to remain the most unrealizable of chimeras.
This, however, is not the case. By pursuing my researches on the whole of the radiations designated by the name of Black Light, I was led to establish: (1) that the luminous rays, or at all events certain of them, passed without difficulty through a large number of opaque bodies; (2) that the invisible rays which pass through them can be easily rendered visible.
If, then, our eye does not see through opaque bodies, it is not because luminous rays do no pass through, but because our retina is insensitive to these rays. If the eye of the lynx does not really possess the property conferred on it in the old legends, there is no scientific reason why it should not. It would be very easy to imagine an eye but little different from ours, and, moreover, possibly possessed by nocturnal animals, which should have the property of seeing through opaque bodies.
Figure 30 ~
It is this artificial eye, sensitive to radiations invisible to the human retina, which is realized by the following experiments.
By means of a heliostat and a lens a pencil of light is conducted on to the collimator of a direct vision prism, and the spectrum is received, in the dark, on a screen of zinc sulfide gradated in wavelengths according to the formula of the prism used. This screen is previously rendered sensitive by a short exposure to light.
After this exposure and before causing the spectrum to act on the screen, a portion of the part on which it is to fall is covered by a layer of the opaque body of which it is desired to study the transparency -- say a sheet of black paper. By cutting off the spectrum after a moment and displacing the opaque strip, we immediately seem by the partial blackening of the phosphorescent screen under it, the region of the spectrum which has passed through it. It extends from 0.8 to 3 microns.
Figure 31 ~
Figure 32 ~

Now let us examine how, by utilizing the properties of these radiation, a body enclosed in an opaque case may be rendered visible. We have simply to follow the explanations given below the above figure. In a few seconds the object enclosed in a box is seen outlined on the screen which covers it.
The luminous source of which the rays pass through the opaque body is a paraffin lamp covered with black paper. The operator is therefore in complete darkness, in the midst of which there appears on the screen the objects contained in the opaque box.
Of the various experiments realizable by the above process, the most striking is that of the visibility of an object enclosed in a box. This being placed in front of the invisible lamp, we see gradually appearing out of the total darkness in which the operator is the image of the enclosed object. When working with transparent screens of large size the effect is surprising.
The invisible luminous rays are much less penetrating than the x-rays, and can in no way claim to take their place.
At the time of my first experiments in photography through opaque bodies, I was not aware of the sensitiveness of zinc sulfide to the infrared rays, and I made use of photographic plates rendered sensitive to radiations of great wavelength by having them previously made cloudy. The exposures then lasted hours instead of seconds. I reproduce later (Figure 40) one of the images thus obtained.
The above experiments needing a little attention and some care, I have sought to supplement them by others which only demand an infinitesimal amount of attention and no apparatus. The following one enables a body enclosed in an opaque receptacle to be seen:
A sheet of black paper is fixed on a glass plate, and on its surface is fastened a cross cut out of a thin strip of tin. This cross is covered with a second sheet of black paper, so that it is imprisoned between two sheets of this opaque paper. We now have to make it visible.
A screen of zinc sulfide on cardboard is illumined by daylight and its surface applied against the strip of glass covered with black paper. The face next the phosphorescent screen is exposed for 10 seconds at a distance of 20 cm from a paraffin lamp. The whole affair is then carried into the dark room. On lifting up the phosphorescent screen there will be seen on its surface the image of the metallic cross which was enclosed between the two sheets of black paper.
The above experiment is, moreover, the one I have recourse to in order to verify at once the transparency of the bodies, black paper, ebonite, etc., employed in my experiments, some samples of which turn out to be opaque by reason of the foreign bodies they contain.
The zinc sulfide with green phosphorescence, being rarely met with in commerce, one can at a pinch replace it, in the case of the last described experiment only, by a screen of calcium sulfide; but it is indispensable that there should be an interval of at least 24 hours between the exposure of the screen to daylight and the time when the experiment is effected. If a screen recently insolated, and consequently very luminous, is used, no image is obtained on its surface.
[Green-phosphorescent ZnS is caused by the presence of ~ 0.01% copper activator. -- Ed.]
When these experiments were first published I gave away the little material enabling them to be repeated to all the learned men who asked me for them. A distinguished professor pf physics, Prof Izarn, wrote to me: "I hastened to effect the experiment with the material you were good enough to send me, and I was stupefied with the clearness of the results. I should never have believed it would be so evident and so rapid". It must be acknowledged, however, that the majority of physicists preferred to deny the exactness of my experiments rather than to repeat them. Others tried them with the aid of a photographer’s red lamp, which instantaneously extinguishes the phosphorescence of the zinc sulfide, and, naturally, observed nothing.
2. Photography through Opaque Bodies ~
It has been explained above (Figure 31) how objects enclosed in opaque boxes may be photographed. We will now vary these experiments by photographing external objects, such as a house, through an opaque body.
For the realization of the experiments which now follow, various objects can be used. The most convenient is Japan varnish, which is poured two or three millimeters thick on the movable diaphragm of the focusing tubes, for the back of which is substituted a thin plate of mica fixed to the sides by strong glue. When the varnish is dry it will be observed, by interposing it between the sun and the eye, that it appears absolutely opaque. The object is to be reproduced on the ground glass of the camera is focused, and the diaphragm is put in front of the focusing tube. It thus constitutes an opaque body placed between the light and the roughened glass.
A screen of zinc sulfide is then illuminated by daylight, and placed in the usual dark slide of the camera as if it were an ordinary photographic plate. Then lifting in the ordinary way the shutter of the frame, it is left open before the object to be reproduced for a period varying with the light.
Figure 33 ~

The photograph here given (Figure 33) is that of a house in daylight, and the objective used was a portrait lens. The exposure lasted one minute.
The exposure at an end, the frame is closed and taken into the dark room, whence care has been taken to eliminate all (especially red) light. On opening the frame, we see on its surface the image of the object which was placed in front of the objective. In order to fix it, the phosphorescent screen is placed, while still in the dark, for 5 minutes against the surface of a gelatino-bromide plate, which is afterwards developed in the usual way. We have an image obtained by using the invisible rays of light. I give here (Figure 34) an image thus obtained.
The want of clearness comes (1) from the focusing with great wavelengths only being possible by calculation; (2) from the phosphorescent screens used as sensitive plate having a rough surface.
I said above that it was possible to employ very different substances as opaque bodies, but it must be remarked that with a ground glass no better image could be obtained than by placing a transparent ground glass before a focusing tube. Unpolished surfaces play, as is known, the part of diffusing screens. If we wish therefore to use as opaque body unpolished or badly polished matter, it is necessary to place it, not in front of the objective, but immediately in front of the phosphorescent screen -- that is to say, in contact with it.
It is useless to try the preceding experiment with calcium sulfide. The body is so little sensitive to radiations of great wavelength that even with an hour’s exposure no image would be obtained.
Some of the experiments just described -- that is, those where the object to be reproduced is enclosed in an opaque box interposed between the sulfide screen and the light source -- succeed just as well with the light of a paraffin lamp or even of an ordinary candle, as with sunlight. This does not result only, as might be supposed, from the richness of artificial sources of light in infrared radiations. It is especially due to the fact that the brightness of the objects lighted by reflection, as is the case with all those examined in daylight, is immensely less than that of the source which lights them. An object brought very close to a candle has very little luminosity, but the flame of the candle itself is extremely luminous.
Its radiancy is superior to that of a white wall lighted up by the sun at full noon in the month of August. This is shown by placing the candle against the wall, when the flame is brighter than this last.
The fact that the brilliancy of a simple candle, or even of a modest match, should be ore intense than that of an object lighted by the sun having seemed inadmissible to many persons, I took some instantaneous photographs (Figure 35) of a candle enclosed in a lantern which almost entirely enveloped it, and at the top of which was a white cardboard lighted by the sun. When developed, we recognize that the image of the candle is more intense than that of the cardboard.
This intensity of sources of light, notwithstanding their feeble illuminating power, may be shown likewise, by photographing instantaneously, and at night, a street lighted by gas lamps. None of the objects lighted up will appear in the photograph, but all the gas lamps will be reproduced.
These observations allow us to understand the experiments which follow relating to instantaneous photography in the dark through opaque bodies.
Figure 33 ~

Figure 34 ~

Figure 35 ~
3. Instantaneous Photography in the Dark ~
The experiment about to be described allows us to photograph in 1/30 second the image of a luminous source (a candle enclosed in an opaque box), then observer being himself in complete darkness.
With a camera furnished with a wide focus-tube and a so-called instantaneous diaphragm, we focus in the proper position on the ground glass -- so as to have an image of about equal size to the original -- the flame of a candle or of a small paraffin lamp enclosed in a laboratory photographic lantern. The lantern is then closed with the opaque body chosen -- black glass, ebonite, or Japanese varnish -- fixed between two strips of glass. The observer is consequently in complete darkness. We then introduce into the dark slide of the camera a screen of zinc sulfide illumined by daylight, and we uncover the objective for the thirtieth of a second. On opening the slide in the dark there is seen on its surface the image of the source of light, which may be preserved by placing it against a photographic plate. The photograph represented here (Figure 37) was thus obtained.
If the observer wishes to see the image form before his eyes, he has simply to use one of the transparent glass screens before mentioned, and open the back shutter of the dark slide to as to be able to observe what passes on the screen during exposure. The screen is transparent enough to show on its back the image formed on the front face.
This experiment shows the astonishing sensitiveness of zinc sulfide with green phosphorescence to radiations of great wavelength, a sensitiveness approaching that of gelatino-bromide for visible light.
Calcium sulfide does not allow this preceding experiment to be realized, nor do the sulfides of zinc with yellow or red phosphorescence.
Figures 36 / 37 ~

4. Transparency of Different Bodies to Infrared Radiations ~
The transparency of bodies to radiations of great wavelength is never complete for the same body. That which we observe with the visible spectrum is equally true with the invisible. Transparency is always selective for each substance. The bands of transparency always border on those of opacity.
There would have been no great interest, and it would have taken an extremely long time, to determine the regions which were transparent for each body examined. The transparent region determinable for sulfides goes from the extremity of the visible spectrum -- i.e., 0.8 microns -- up to 3 microns or thereabouts.
In a general way, it may be said that the infrared radiations are more penetrating than those of the visible spectrum. Although not by any means a constant law, it is noticed that transparency seems to diminish as the wavelength is reduced. It is known that light is less penetrating the farther it advances towards the ultraviolet. At the extremity of this region, all bodies, even a glass plate 1/10 mm thick, become opaque, and to waves of the length of 0.1 micron a stratum of air 1 cm is as opaque as lead.
But, I repeat, there is nothing absolute in this law. The transparency also depends on the structure of the bodies traversed. There are some which are very opaque for great wavelengths. It is for this reason, for example, that the atmosphere absorbs all radiations above 5 microns, and this is the reason why these latter do not figure in the solar spectrum. The radiations of higher magnitude -- that is to say, from 5 to 60 microns -- are only observed in flames, and most bodies have a rather low transparency with regard to them. The Hertzian waves, supposed to be analogous to light, on the contrary, easily traverse bodies which the great light waves do not penetrate.
Bodies heated to a temperature not very high -- that is to say, under 100° C -- emit radiations hardly exceeding a length of 5 to 6 microns. They have also but little penetration. Melloui in the course of his experiments observed the fact, which was quite inexplicable in his time, that transparent bodies, like glass or quartz, were opaque for the waves emitted by a body heated to 100° C.
It will be remembered that this opacity is utilized in greenhouses in which glass bell-shades shelter certain plants. The visible waves of light pass very freely through the glass and heat the bodies on the inside of it. The latter become at once sources of radiant heat; but owing to their length, the waves emitted are unable to escape through the glass. They are thus imprisoned, and the plants do not become chilled.
Radiations of great wavelength only appear to act on phosphorescent screens up to 3 microns or thereabouts. Screens may indeed be impressed at a greater distance, i.e., up to and beyond 6 microns, but by a very different mode of action to that utilized up to the present. The rays then act only by their thermal properties, and that is why their action only manifests itself at the expiration of some 30 seconds. The effect produced may be observed by covering a part of the screen with a strip of glass -- opaque to these radiations -- so as to have a field for comparison. A screen of slightly luminous zinc sulfide exposed for 30 seconds to the radiations emitted by a body heated to 50° C becomes slightly impressed. The radiations here act, I repeat, by their thermal action, and not by the specific action on phosphorescence utilized in the previous experiments.
My method of observation of the transparency of bodies by means of phosphorescence is very simple. On one half of the transparent zinc sulfide and glass screen is placed the body serving as unit of comparison -- for instance, a strip of chlorite 1 mm thick -- and on the other, the opaque body of which it is desired to ascertain the relative transparency.
I then take up a position at a fixed distance from a paraffin lamp placed in a dark room, the screen of zinc sulfide having been isolated by exposure to daylight. Its surface is protected by a metal plate, and when I am at a suitable distance from the lamp -- 1 meter for example -- the half not covered by ebonite is uncovered, and the light allowed to act on it for a given time -- say, 5 seconds. Then covering up this part, and uncovering that over the opaque body of which it is desired to ascertain the transparency, we allow the rays of light to act until the intensity of the two surfaces is the same. If it takes 20 seconds to obtain this identity, I conclude that the body under experiment is twice as transparent as the ebonite taken as standard. As phosphorescent sulfides receive cumulative impressions, the transparency becomes a function of the time. We must then take the time as its ratio -- which is what the method just indicated does.
Table of Transparency of Various Opaque Bodies to Invisible Radiations Not Exceeding 8 Microns ~
Black Japanese Varnish: even at a thickness of 1 cm, is transparent to such a degree that the rays passing through it impress the phosphorescent screen in 2 seconds. Even when less than 1 cm thick, it produces the impression in 2 or 3 seconds.
Pure Ebonite: nearly as transparent as varnish up to 1 cm
Ebonite: half a mm thick, containing 1% lamp-black or metallic oxides; almost completely opaque.
Black Paper: very transparent, but only half as much so as pure ebonite.
Red Phosphorus in plates 1 mm thick: as transparent as ebonite.
Wood, Stone, Marble, Grey Cardboard, Black Cloth: Transparent, but much less so than ebonite.
Colored Glass: Very transparent, but red the least and orange the most so for the infrared.
Bromine and Iodine: Still more transparent than ebonite.
Fused Silver Chloride: cut into plates less than 1 mm thick; slightly less transparent than ebonite.
Antimony, Lamp-Black, and Arsenic: very opaque.
Ammoniacal Copper Sulfate, Potassium Bichromate (saturated solution), Water-Glass, and Alum (1 mm thick): transparent, but less so than ebonite.
Iron sulfate (saturated solution, 1 cm thick): one-fourth as transparent as ebonite.
The above shows that bodies formerly considered opaque for infrared radiation, such as alum, black paper, water glass, etc., possess, on the contrary, great transparency.
When we wish to compare very rapidly the relative transparency of certain bodies, the following process may be employed: the substances to be examined are cut into bands and fixed on a glass plate, and then placed on a phosphorescent screen of brilliant zinc sulfide. They are exposed for 3 or 3 seconds in the dark to the radiations of a paraffin lamp. The darker the shade of the sulfur under these bands, the greater the transparency of the bodies.
Of all the bodies enumerated above, the one found most opaque is lamp-black or the substances containing it. Black paper and ebonite which contain it -- and that is frequent -- at once become opaque. Before using them in experiments, it is therefore essential to examine their transparency, which only takes a few seconds.
The opaqueness of lamp-black enables us to easily realize the following experiment, paradoxical at first sight though it is, viz., to reproduce, either by contact or by means of a photographic camera, a print placed in an envelope of black paper, and shut up in an ebonite box. He printing ink, which contains lamp-black, is not traversed, while its surrounding are, I have given in Figure 34 a photograph thus obtained.
This opaqueness of lamp-black allows some of the rays to pass, but not the whole of them, since it arrests all those comprised between 0.6 and 3 microns. I have often repeated that throughout the visible, as well as the invisible spectrum, transparency is always selective -- that is to say, that an opaque body is, like a colored glass, only transparent for certain radiations.
6. Use of the Invisible Rays for Rendering Visible Dark Bodies at a Great Distance ~
When I published for the first time some of the experiments related in this chapter, the Minister of Marine of that time asked me whether it would be possible to mask, with an opaque body, the man-of-war or of a lighthouse, in such a manner as to render them invisible to the enemy and yet visible to those of one’s own vessels which were furnished with suitable apparatus.
The solution to the problem was simple enough, but it has lost all practical interest at the present time, since the progress of wireless telegraphy which enables distant vessels to communicate with one another, and I therefore think it can do no harm to publish details of my experiments. Generalizing the problem, I sought to discover whether a ship could not project into a harbor, a fortress, or a town, rays of light invisible to the besieged, but visible to the besiegers, thus allowing a precise aim to be taken at the enemy while keeping the gunners invisible.
The following problem therefore was set for solution: To render visible without rendering it luminous any obscure body -- for instance, a vessel with its lights out in a dark roadstead.
We must first consider that, when we project on to a dark body a pencil of visible light, this body is only rendered visible by the rays that it reflects. We know, on the other hand, that about 99% of the radiations projected by the best sources of light are quite invisible, and therefore unusable. So that, when we find the means of separating the visible from the invisible radiations, we shall only deprive our light of 1% of its total emission. The greater part of it will, consequently, still remain.
The screens mentioned in the above possess this very property of eliminating the visible and allowing the invisible rays to pass. If a pencil of this invisible light is projected onto a dark body, this body will reflect it as it would ordinary light. It would therefore appear luminous to an eye capable of perceiving the invisible radiations reflected by it. Such an eye does not exist, but a phosphorescent zinc sulfide screen can take its place.
Instead of the ground glass of a camera furnished with an objective of small focus capable of embracing a wide extent of the horizon, let us expose a screen of zinc sulfide previously rendered phosphorescent by means of a ribbon of magnesium r by the x-rays from a Crookes tube; there will then appear on its surface the image of the bodies in darkness, on which has been projected a pencil of invisible radiations.
This invisible light will simply come from the reflection of that sent by the electric searchlight carried by all warships, the visible rays of which we shall have previously masked by means of an opaque plate.
This plate must not be made of ebonite or black paper, as used in our preceding experiments, for the reason that they would be promptly destroyed by the heat. The only available body is black glass, of which there exist varieties opaque enough to blot out the disc of the sun when interposed between that orb and the eye. Not that all black glasses by a long way are transparent to invisible radiations, but there are some easily obtained in commerce. It is only necessary to ascertain their transparency by the means I have described.
All the experiments set forth in this chapter are based on the use of bodies sensitive to radiations of great wavelength, but this sensitiveness is only very great for radiations hardly exceeding 3 microns. Now, those emitted by bodies at a relatively low temperature -- the human body, for example -- are of much greater wavelength, and do not impress phosphorescent matter. If we could discover a body sensitive to those radiations, nothing would be easier than to photograph a living body in the dark without any other source of light than the invisible light it is continuously emitting.
Down to the absolute zero of temperature, all bodies incessantly radiate, as has been seen, waves of light invisible to our eyes, but probably perceptible by the animals called nocturnal and capable of finding their way in the dark.
To them, the body of a living being, whose temperature is about 37° C, ought to be surrounded by a luminous halo, which the want of sensitiveness of our eye alone prevents our discerning. There does not exist in nature, in reality, any dark bodies, but only imperfect eyes. All bodies whatever are a constant source of visible or invisible radiations which, whether of one kind or the other, are always radiations of light.
The Part Played by the Various Luminous Radiations in the Phenomena of Life
1. The Part of Light in the Phenomena of Life ~
As the invisible infrared rays form the greater part of the solar spectrum, it may be imagined that they play a considerable part in meteorology and in vegetable physiology. Their properties in this respect are very little known. Hitherto, their caloric actions which were long since observed, and their power of passing through a great number of opaque bodies as brought to light by my researches have alone been studied.
It occurred to me to also study some of the physiological actions of the infrared rays -- that is to say, their influence on vegetable life, and to inquire especially whether they might not exercise some of those antagonistic effects established during the study of phosphorescence which will be studied in detail in the next chapter. For want of materials, I was unable to proceed very far in these researches.
We know that visible light has two opposite actions on the life of vegetables -- the one oxidation or the respiratory function, the other reduction or the chlorophyllic function.
The first of these may be accomplished in the dark. By its means the pant absorbs oxygen and exhales carbonic acid, as do animals.
The chlorophyllian function, the converse of the above, on the contrary, can only take place in the light, and is due solely to absorption. Thanks to this, the plant decomposes carbonic acid and fixes the carbon in its tissues. The luminous energy stored up by the chlorophyll enables the protoplasm of the plant to transform mineral substances into those organic products, complicated and charged with energy, without which the life of the higher animals would be impossible. Vegetables thus establish a permanent link between the mineral and the animal world. Thanks to them, matter passes without ceasing through different forms of life rising progressively from the mineral to the higher animal.
In this perpetual cycle, two elements of transformation, bacteria and chlorophyll, play a preponderant part. The bacteria bring back to the mineral state the products used by the functions of the higher lives, and the chlorophyll raises the mineral substances to the organized state.
Bacteria are able to pursue their destructive action in complete darkness. Chlorophyll is obliged to absorb the luminous vibrations before playing its part. The vegetable world therefore represents a transformation of light. It is the luminiferous ether, absorbed and transformed by plants, which ripens our harvest and makes green out forests. Life represents one of its transformation.
It cannot, however, be said that the very great energy stored up by the plant is entirely due to the very slight energy produced by the absorption of the luminous rays by the chlorophyll. The rays absorbed, no doubt, act by provoking liberations of intra-atomic energy, the mechanism is which is not comprehended. The vibrations of the ether probably release forces which thousands of ages have in times past accumulated within the atom.
What effect have the various visible or invisible radiations on the life of vegetation? The part played by the first named has been studied by several generations of seekers. That of the second is largely unknown hitherto by reason of the insufficiency of the methods employed to determine its action.
2. Methods of Observation of the Action of the Solar Spectrum on Plant Life ~
The value of experiments always depends on the choice of methods. Those employed to study the actions of the various parts of the solar spectrum, especially the infrared rays, on plant life are unfortunately marred by causes of error which take away all value from many of the results -- contradictory moreover as these are -- obtained up to the present time. It is easy to put these in evidence.
To observe the properties of the various radiations it was natural to think of decomposing light by a prism, and of placing the plants under the various radiations thus separated. The decomposition of carbonic acid by the leaves, for instance, is measured by introducing them under the different pencils of light which the prism has separated.
This method, so simple in appearance, gives rise to causes of considerable error. The first and most serious is that the light, when sufficiently dispersed to spread over a certain surface, loses its intensity to an enormous extent. Now, the influence of intensity in chemical reactions is, as has been shown, of capital importance. When operating with a prism one is inevitably led to seek in the color of the ray the cause of effects due, in reality, to differences of intensity. It has long been known that in the faint light of a room plants decompose very little or even no carbonic acid at all. Yet this light contains the rays necessary for its decomposition. The light fails to produce this only through lack of intensity.
This first cause of error would of itself be sufficient to vitiate all the consequences drawn from the facts observed. Moreover, it is not the only one. Prisms, especially those made from flint, generally chosen on account of their great power of dispersion, absorb nearly all the ultraviolet rays, the action of which is very important, and a great part of the infrared.
No doubt these two drawbacks can be theoretically remedied by using quartz or rock-salt prisms, which in fact has been done. But the dispersive power of these substances is very small, and their spectrum consequently is small in extent.
The causes of the preceding errors, and of numbers more of which the complete details would be too technical to be given here, explain sufficiently the divergences in the results obtained by former observers. According to some, the decomposition of carbonic acid by the plant -- that is to say, its most important function -- is nil in the red, and considerable in the green. According to others, it is exactly the contrary -- the action is nil in the green, and at its maximum in the red. This last result is, however, the most probable, seeing that it is in the red and its neighborhood that are to be found the bands of absorption of the chlorophyll. To sum up, there is little exact information to be gained from the studies effected by the use of prisms.
The results obtained by replacing the prism by colored glass are no better. This is a pity, for the process is practically very simple, since all that one has to do is cover over the greenhouses in which the experiments are conducted with panes of glass of different color.
This method is the result of an error into which many experimenters have fallen. Because the eye sees but one color through a colored screen -- blue glass, for instance -- it is imagined that this screen only allows the one color perceptible by the retina to pass through. Now this is not the case at all. No glass, except the red, is monochromatic. All allow the whole of the spectrum to pass through. This is easily verified by holding colored glass between the sky and the slit of a small direct-vision spectroscope. I have had occasion to examine a considerable quantity of colored glasses, and have observed that all except the red allow the whole of the spectrum to pass, and only weaken the relative intensity of its various parts.
By covering a greenhouse with colored glass, then, we do little but reduce unevenly the intensity of the various luminous rays. When a plant is placed under colored glass, it is almost the same as placing it in a badly-lit room.
The use of colored glasses to separate the various radiations carries with it yet other sources of error. As they absorb the infrared rays very unequally, we may attribute to the influence of light what is due to that of heat. Between one glass and another, the differences of caloric action are considerable. By placing a thermometer in boxes with a capacity of about one cubic decimeter, each one covered by different colored glass and thus forming small glass-houses, I observed that the internal temperature, which in the sun was 30° C, went up to from 10° to 15° in 10 minutes, according to the temperature of the glass.
The only experiments realizable with colored glass are those made with red glass. This is, as has been said, almost monochromatic.
The experiments effected in greenhouses covered with such glass at the observatory of Juvisy seem to prove that a certain number of plants receive in red light a much more considerable development than in ordinary light. Such, for instance, is the case with sensitive plants, lettuce, gladiolas, geraniums, begonias, potatoes, male ferns, etc. Some (beetroot, pansies, wallflowers, etc.) on the contrary, thrive less under it.
Were we to admit as demonstrated that certain plants develop much better in red than in white light, the conclusion must naturally be adopted that, as in the case of phosphorescence, certain rays have a contrary effect to that of others. In white light, a plant evidently receives as many red rays as under a red glass, since this last simply eliminates from the light all the rays saving the red. If the fact of this elimination alone favors in large measure the development of the plant, it is because the eliminated rays act so as to weaken the action of the red. This would be exactly what is observed with regard to the photographic plate, as we shall see in the next chapter. This plate becomes much more luminous when we withdraw certain antagonistic rays from the light which strikes it.
The antagonistic actions observed as regards phosphorescence thus exist also for plant life. Green has already observed that the violet and the ultraviolet rays tend to destroy the diastase, while its production increases under the influence of the red. It will be seen further on that, in experiments made by me, the infrared destroys the green matter in plants and other substances formed in the luminous part of the spectrum.
3. New Method of Studying the Physiological Action of the Infrared, and Results Obtained ~
In the preceding researches, hardly anything but the visible rays of the spectrum have been dealt with. The only means known in former times of studying the infrared rays being separation by the prism, and as this collects the radiations of this end of the spectrum on a very restricted surface, we could scarcely determine their action.
The fact, brought to light by my researches, of the very great transparency of black paper, ebonite, etc., to rays of great wavelength, enables them to be easily separated from visible light. A greenhouse is simply covered over with one of these substances -- for choice, the black paper. It is thus plunged in utter darkness, but bathed with invisible light. In reality, the greenhouse has only by this means been deprived of about a tenth of the total light (visible and invisible) which it would have received from the light of the sun.
It probably need hardly be remarked, that there can be no comparison made between an enclosed place in which there only penetrate dark radiations of great wavelength and the cellars in which the action of darkness on plant life was studied. The darkness of the greenhouse is the same to the eye as that of the cellar, but the effects produced must necessarily be very different, since the greenhouse is bathed in the waves of an invisible light which the cellar does not contain.
I have, unfortunately, not been able to pursue my experiments on this subject for a long time, the garden in which they were carried out only having been placed at my disposal for one season. They may, perhaps, render service to horticulturists by giving them the means of modifying the color of certain plants, and the taste of different fruits.
It may be asserted in a general way that the infrared up to 2 or 3 microns destroys the green matter formed under the action of light and also certain coloring matters, while it reduces the quantity of sugar, suppresses the sapid matter, and thus transforms the taste of different parts of the plants.
Here is, however, the summary of my trials: --
(1) Seeds of various plants ~ Lettuces, cucumbers, grains, etc., set to germinate under glass bellglasses, covered with black paper, transparent to the infrared, all germinate quicker than by the light of the sun and then wither and die in abut a fortnight.
(2) Plants developed in the light of day, and then exposed to the black light ~ The various plants behave very differently. Here are a few examples: Reine marguerite does not flower. Begonia withers in about 10 days. Strawberries are not modified, and ripened very well. Cucumbers and haricots die from the withering of their leaves.
(3) Fruits and different parts of vegetables ~ Artichoke tops enveloped in black paper, transparent to long radiations, blanch completely in a few days, but develop better than their neighbors exposed to daylight, while gaining much in quality. Pears, peaches, and grapes blanch somewhat but develop very well. They were covered up as soon as they began to form. These three forms of fruit present the peculiarity that they lost a part of their sweet taste and their aroma. Tomatoes lose their red color, and become completely white.
I repeat that I only give these experiments as general indications. In fact, they deserve the criticisms which could have been avoided had I been able to repeat them. The comparative method, indispensable for drawing conclusions from experiments in which several factors may operate, demands that there should be only one condition varying from one experiment to another, so as to be able to attribute differences in results to the difference of condition alone. Now, in these experiments, we have not always taken into account aeration, heat, etc.
As the different parts of the light exercise, as we are about to see, strongly antagonistic actions, it would be very useful, both in the light baths now much used in medicine and in biological researches, to be able to separate the rays so as to study the part played by each of them.
In the present state of science, the comparative studies which are possible on the action of the various rays of visible or invisible light, without seriously reducing their intensity, are limited to the use of the following screens: --
(1) Entirely without screen ~ The parts which act (the whole of the solar spectrum) range from 5 microns to 0.295 microns.
(2) Screens formed of thick window glass ~ The greater part of the uv rays and the ir rays beginning at 2 microns are suppressed.
(3) Screens formed of red glass ~ Complete suppression of all the uv and of all the visible spectrum as far as the red. The only rays which act are the red and the infrared as far as 2 or 3 microns, according to the quality of the glass.
(4) Screens formed of black paper or ebonite ~ Suppression of all the visible spectrum. The active rays are infrared.
(5) Screen formed of a strip of metal ~ No ray traverses the metal, but it becomes heated, and emits from its underside radiations of 6 microns and beyond, according to its temperature. These radiations do not exist in the solar spectrum, but it would be of interest to ascertain their action, and discover whether it is simply caloric.
Notwithstanding their insufficiency, these researches give us a presentiment of the great interest of the researches for which, through lack of a suitable laboratory and of sufficient means, I have only been able to sketch the way.
The Antagonistic Properties of Certain Regions of the Spectrum
1. Rays which Illuminate and Rays which Extinguish ~
The study of the infrared has led us to observe that it often exercises actions diametrically opposed to those of the other extremity of the spectrum, destroying, for instance, an action produced under the influence of this last.
The discovery of these antagonistic actions of the two extremities of the spectrum is contemporaneous with the origin of photography; but we are compelled to imagine that the experiments effected to prove this phenomena were not sufficiently demonstrated since their interpretation has log been disputed, and was again challenged recently in a long discussion before the Societe de Physique. The slow actions of inversion following one another in photographic impressions seem, in fact, to lend themselves to various interpretations. The question, evidently, can only be entirely elucidated by finding means of rendering instantaneously evident these antagonistic actions. It is these instantaneous effects which are realized in the experiments which follow.
The first observations on phosphorescence made it plain that all one end of the spectrum, comprising the blue, violet, and ultraviolet rays, illumine a phosphorescent screen taken out of the dark. The other end of the spectrum (the green, red and infrared rays) on the contrary, extinguish phosphorescence, and never produce it. Certain rays therefore act as illuminating and others as extinguishing rays. These two actions are plainly antagonistic but as they are very slow in the case of most phosphorescent bodies, they lend themselves to various explanations. To put them in evidence, we needed a body extremely sensitive to those radiations which extinguish phosphorescence. Zinc sulfide with green phosphorescence is the only body actually known to possess this property, and all my experiments have been made with screens coated with this sulfide in the manner described in a former chapter.
If it is exact that certain rays produce phosphorescence, and that others acting in the opposite direction consequently extinguish it, it is evidence that by depriving the light of these extinguishing rays by the interposition of suitable screens, we may increase the brilliancy of the phosphorescence on a given screen. We shall see that this is really so, and that, for example, a body which does not become illuminated behind a glass trough of quinine sulfate, will become so if we keep back, by means of appropriate screens and without changing the position of the trough, certain rays from reaching its surface.
E. Becquerel formerly pointed out that a screen of phosphorescent sulfide exposed to the light behind a trough of quinine sulfate, did not become illuminated, and he attributed this phenomenon to the absorption of the ultraviolet rays by the quinine sulfate -- rays which, according to him, "are the principal ones to excite phosphorescence". This explanation is wholly insufficient, for if the ultraviolet rays are able to excite phosphorescence, they are not by any means the ones that excite it most. Phosphorescent bodies are much better illumined by the blue and violet rays -- that is to say, by the part of the spectrum comprised between the lines G and H. This point must be borne in mind in order to understand thoroughly the experiments which follow.
By varying the duration of the exposure of the phosphorescent screens we can easily take note of the sensitiveness of the sulfides in the various regions of the spectrum. With the projection spectroscope and the condenser above described (Figure 21), a screen of calcium or zinc sulfide is impressed in four seconds between G and H, and not at all in the ultraviolet. There must be an exposure of several minutes to obtain an impression in this last region. On the blue side the prolongation of the exposure extends the impression nearly up to the line F, but no farther.
Let us now take a screen of zinc sulfide, a body so little sensitive to violet rays that it does not light up at all behind the trough of quinine sulfate. We will now compel it to be brightly lit up behind this trough by simply superposing on the latter another trough which does not arrest the blue rays, but does stop the extinguishing green, yellow, red and infrared rays.
This experiment, and others of the same order showing distinctly the instantaneous action of the extinguishing and illuminating rays, are decisive. For lecture purposes they amy be thus simplified.
We expose to sunlight (1) a screen of zinc sulfide, and (2) by the side of it another screen similar, but placed behind a flat-sided flask filled with a saturated solution of ammoniacal copper sulfate. This solution is almost opaque to the eye when in a stratum of about 2 cm. Leaving the flask in its screen, it is taken with the other into the dark, and then it will be observed, after withdrawing the flask of copper sulfate, that the sulfide screen placed behind this last, notwithstanding the apparent obstacle it presented to the passage of light, is much more luminous than the screen directly exposed. This difference is merely due to the fact that the copper sulfate has absorbed the extinguishing rays, and only allowed the illuminating ones to act. On the screen exposed directly to the sun the brightness of the phosphorescence is much less because a part of the effect of the illuminating rays has been destroyed by the extinguishing rays mingled with them.
We may now return to the description of the experiment with the quinine sulfate trough, behind which we can light up or not at will a screen of phosphorescent sulfide. No experiment shows more strikingly the parts played by the extinguishing and by the illuminating rays.
Behind a flat flask containing a perfectly clear 10% aqueous solution of quinine sulfate, acidified by sulfuric acid to the point of complete solution, we place a screen of zinc sulfide and expose the whole to the sun. Notwithstanding the complete transparency to the eye of the quinine sulfate, and however prolonged may be the exposure, we observed on taking the flask and screen back into the dark that the zinc sulfide exhibits no trace of phosphorescence. This absence of phosphorescence is due solely to the fact that as the quinine sulfate retains a part of the illuminating rays and allows all the extinguishing ones to pass, it is these last which prevail.
Figure 38 / 39 ~
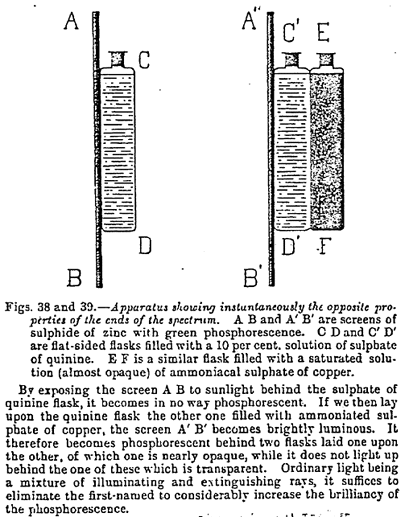
To prove that this is really so, we will again expose to the sun our zinc sulfide screen, placed behind the trough of quinine sulfate; but in front of this latter we will again place the trough of ammoniacal copper sulfate. Then, taking the trough back into the dark, we observe that our sulfide screen is brilliantly illumined, although it has only received the light through an almost opaque liquid.
The explanation is easy. The quinine sulfate held back, as I said, a part of the illuminating rays and allowed to pass all the extinguishing rays; accordingly, it prevented all phosphorescence. By placing in front of it a trough of copper sulfate we suppress the great majority of the extinguishing rays (red and infrared) and consequently the blue and violet illuminating rays are allowed to act. The sum of the power of the illuminating exceeding that of the extinguishing rays, the screen becomes phosphorescent.
If we had replaced the copper sulfate trough by a blue glass placed in front of the quinine sulfate, we should still have had illumination, but weaker than with the copper sulfate, because the blue glass stops the extinguishing rays very insufficiently, especially those of the infrared region.
What has just been said regarding the action of the extinguishing and illuminating rays on phosphorescent bodies allows us now to comprehend the part taken by the screens interposed between phosphorescent bodies and the sources of light.
Light being a mixture of radiations capable of acting in contrary directions, and its composition differing greatly with the sources employed, or with the filtering screens interposed between the light and phosphorescent bodies, the explanation of the facts I am about to enumerate will be easily arrived at.
(1) A screen of zinc sulfide is much more illuminated in the dark than in sunlight.
(2) The same screen is more illuminated under a blue glass in the dark than in sunlight.
(3) Behind a trough 2 cm from back to front, containing an ammoniacal solution of copper sulfate, the illumination of the screen is still more intense, but on this occasion it becomes brighter in the sun than in the shade.
(4) Behind a trough of alum or of iron sulfate the illumination of phosphorescent bodies is reduced instead of being increased.
(5) By the light of a paraffin lamp or of a candle the illumination of a screen of zinc sulfide is almost nil, and on presenting to these sources of light a screen insolated by daylight, it at once becomes extinguished. The same phenomenon is not observed with a screen of calcium sulfide.
Here are a few figures giving the luminous intensity of a screen of zinc sulfide illumined under most of the conditions we have just examined.
The brightness of the sulfide insolated by the sun without any screen interposed is about 0.002 B (1/5000 candle power), c.p.). Taking this intensity as our unit, we see in the following table the increase in brilliancy produced by the interposition of various screens between the source of light and the sulfide they show us, for example, that the brilliancy of the zinc sulfide behind a trough of copper sulfate is 14 times as great (3/100 c.p.) as when it has been illumined in the sun without the interposition of any screen.
Luminous intensity of a screen of zinc sulfide exposed in sunlight ~
Relative Intensity (RI) = 1
Intensity of the same screen illumined in the shade ~ RI = 2
Intensity of the same screen exposed in sunlight under a cobalt blue
glass ~ RI = 7
Intensity of the same screen exposed in the shade under a cobalt blue
glass ~ RI = 9
Intensity of the same screen exposed in sunlight behind 2 cm saturated
ammoniacal copper sulfate solution in copper trough ~ RI = 11
These differences are not observed with other sulfides by reason of their lower degree of sensitiveness to the extinguishing action of radiations of great wavelengths. In order to observe them, then a substance like zinc sulfide, sensitive at once to the extinguishing and illuminating rays, was necessary. Its brilliancy under the influence of light always represents the difference between the action of the first-named rays and that of the second.
We see therefore that it is solely on the relation existing in a source of light between the various radiations of which it is composed that the illumination of a phosphorescent body depends. By varying the relation we vary the brilliancy.
This explains the action of a cobalt blue glass screen. It weakens the extinguishing radiations, and consequently increases the illumination. Copper sulfate, which stops the extinguishing radiations in a much greater degree, likewise greatly increases the illumination. Iron sulfate and alum act but slightly because, although they stop a portion of the infrared, and also a part of the ultraviolet, which is an illuminant, they stop neither the red nor the green, which are extinguishers.
As regards the illumination of zinc sulfide being greater in the dark than in the sun -- a very curious fact which has escaped all other observers -- the explanation is exactly the same. Diffused light is blue light reflected by the sky. From spectroscopic observations of already ancient date, it contains relatively many fewer red rays -- and probably also fewer infrared ones -- than the direct light of the sun. Notwithstanding, therefore, the higher luminous intensity of the sun, the sulfide will be more luminous in the shade. This explanation is really the correct one, for if we withdraw from the solar rays by our copper sulfate solutions which act as extinguishers, the illumination will at once become much more brilliant in the sun than in the shade by reason of the greater intensity of the illuminating rays.
The same reasoning explains why zinc sulfide not only does not become illuminated in the light of a strong lamp, but goes out when exposed to it, if previously illuminated. In our artificial systems of lighting nine-tenths of the rays emitted are extinguishing infrared rays. The action of the illuminating rays is, then, destroyed by them. Even by interposing a thick trough of copper sulfate, only a very weak illumination is obtained because, in view of the predominance of the infrared rays, it is impossible to stop them in sufficient proportion. Moreover, the interposition of this trough much reduces the intensity of the illuminating rays. Now, the degree of phosphorescence of a sulfide is, within certain limits, in direct ratio to the intensity of the source of light which has illuminated it. With feeble sources of light, phosphorescent sulfides so not become saturated, whatever may be the duration of the exposure.
All that I have just said regarding zinc sulfide is not applicable, I repeat, to other phosphorescent sulfides by reason of their slight sensitiveness to great radiations, especially to the infrared, and this is why a screen of calcium sulfide does not become appreciably brighter behind troughs which stop the extinguishing radiations. The action of these last is too slow to combat that of the illuminating radiations which already have had time to act.
The eye is even more insensible to infrared radiations than the phosphorescent sulfides other than zinc. If the sensitiveness of the retina were as it is for this last body, the algebraic sum of the effects produced by radiations acting in contrary directions, it would suffice to interpose suitable screens between the eye and a landscape, for the apparent brightness of this landscape to increase in enormous proportions. It would then be perfectly unnecessary to use electricity to light our streets.
Such different actions from the two extremities of the spectrum are not observed in the case of phosphorescence alone. They also exist in photography, as I shall proceed to show.
2. The Opposite Properties of the Various Regions of the Spectrum and their Actions in Photography ~
If photographic plates had, to the destructive action of the infrared rays, a sensitiveness equal to that of zinc sulfide, it would be possible to greatly increase, as we have done for the latter, their rapidity of impression by placing in front of the camera screens which stop the extinguishing rays.
But in this case, because the photographic plate, although very sensitive to the visible rays, is not so to the invisible, which are thus unable to act. It is easy, however, by bringing in the action of time, to show that the extinguishing rays can destroy the impression on the plate produced by the illuminating rays.
Let us place in a photographic dark slide a plate of ebonite which may be 1 mm thick, but which it is better to reduce to ½ mm, so as to obtain sharp outlines. On or under this plate, we will glue a cross cut out of a sheet of tin. Behind the ebonite plate I will introduce in the dark a gelatino-bromide plate of very fine grain, previously clouded by exposing it for 2 seconds to the light of a candle. I close the frame and expose it for one hour in sunlight. The infrared rays pass through the ebonite and destroy the cloud produced on the plate by the previous exposure. In the part protected by the metallic cross they will not have acted, and that is why, on developing the image, we shall find a reproduction of the metal cross on a very light ground. The ebonite plate may also be replaced by two or three superposed sheets of black paper.
Figure 40 ~

There is no analogy between this experiment and the investigations we obtain by over-exposing a plate lighted by the blue or violet rays. The infrared rays which destroy a photographic impression, never produce one. This property is limited to the infrared, for the red rays can, with a sufficiently long exposure, impress a plate. If, in fact, we prolong the exposure under red glass, the plate becomes first clouded and afterwards automatically unclouded. But in this experiment the phenomena of inversion observed with all radiations, and constituting a very different order of phenomena, come into play. In the visible part of the spectrum, any radiation whatever destroys the impression produced by greatly prolonged exposure -- a fact easy to verify by the spectroscope.
We will summarize in the following table the antagonistic properties of the two extremities of the spectrum.
Table of the Antagonistic Action of the Two Ends of the Spectrum ~
Visible Region of Spectrum ~ Invisible Infrared Region
A. Cannot pass through opaque bodies ~ A’. Passes through most bodies
opaque to the eye, metals excepted.
B. Impresses photographic plates ~ B’. Destroys the impression produced
on photographic plates
C. Produces phosphorescence ~ C’. Extinguishes phosphorescence
D. Produces the majority of reactions on which plant life depends ~
D’. Destroys a great number of reactions produced by visible light. Destroys
especially the coloring matter of plants.
E. Energetically dissociates matter, especially throughout the ultraviolet
extremity ~ E’. Has no dissociating action on matter.
The reasons for these differences cannot yet be sought; if the "How" of things is sometimes accessible, their "Why" is not yet so.
Forces if Unknown Origin and Hidden Forces
Chapter I
Universal Gravitation and Hidden Forces
1. The Causes of Gravitation ~
The description of forces of unknown origin might really be applied to all those we have hitherto studied, since we are ignorant of their essence; but at least we know something of their characteristics and their mode of propagation. On the other hand there exist forces such as gravitation, affinity, molecular action, etc., of which we know almost nothing. For this reason they have been relegated to a special division of this book.
All our knowledge relating to gravitation can be reduced to the following definition; bodies attract one another proportionally to their mass and in inverse ratio to the square of their distance from each other. Of the causes of this attraction, of the manner in which it is propagated, and of the speed of its propagation, we can say nothing.
The discovery of the just-formulated law is, it will be remembered, due to Newton, and cost him long research. Gravity had been known a long time before, and Galileo had perfectly studied its laws. But how far did its actions extend, and did it pass beyond the limits of our atmosphere? Of that every one was completely ignorant.
The genius-inspired glance of Newton saw that weight might extend to the different planets and be the cause of their movements in space. He devoted many years of his life to the verification of the exactness of this hypothesis.
After having discovered the laws of gravitation, Newton vainly attempted to determine its cause. "The reason of the properties of weight", he said, "I have not yet been able to discover". Nor were his successors more successful. One gets clearer and clearer glimpses that weight is due to the relations between the ether and matter, connected doubtless by lines of force; but this is only a more or less vague hint which still escapes the teachings of experiment. It is possible that the gyratory movements of atoms are communicated to the ether, and through it to the different material bodies, thereby establishing an attraction between them. The reciprocal attraction of vortices has at the present day been demonstrated by many experiments. When the gyratory movement of the particle is transformed into movements of translation in space during the dissociation of matter, these particles no longer exercise any attractive action on each other, and consequently cease to be ponderable. This explanation was given by M. Armand Gauthier in a memoir referring to my researches.
Gravitation displays this incomprehensible characteristic, which no other manifestation of energy possesses, of not being arrested by any obstacle. The most delicate researches have shown that no body exists which is opaque to attraction.
Gravitation is a very small force, if we consider only the action of the masses we have at our disposal. But it is a force extremely great for considerable masses. Its power is apparent to us every day in the phenomenon of the tides. Under the influence of the combined motion of the sun and moon, the seas are raised an average height of 1 meter, which represented a weight of 1000 kg per surface meter.
Physicists have been able to say nothing more on gravitation than what is said above. In an important memoir, of which I reproduce a few passages, Prof. Vernon Boys has shown perfectly how inexplicable it remains. "It seems to defy", he says, "all our attempts to abandon the inconceivable idea of action at a distance; for even when we might conceive another mode of action, it is entirely incomprehensible that gravitation should act at a distance without regard to the existence or nature of the bodies in its path, and, as it appears, instantaneously. Moreover, in the actual state of our knowledge, no other physical agent, even among those which depend upon the ether, has any influence over the direction or the extent of the notion of gravitation. The difficulties that we experience in creating a mechanical representation of the ether are considerable; but the mode of propagation of gravitation seems still further out of our reach" (Actes du Congres de Physique de 1900, t. iii, p. 306).
The speed of the propagation of gravitation was estimated by LaPlace as being immensely higher than that of light. M. Poincare considers it as propagated with a velocity of the order of that of the light-vibration.
We do not know how gravitation is propagated, but it seems to me that the law of the inverse square of the distance allows us to imagine gravitic waves having a form analogous to that of light, electric waves, etc. It is, in fact, only to forces which are propagated in this way that such a law is applicable. It is only a consequence of the geometrical properties of spherical bodies, and results simply from the fact that the surfaces of concentric spheres are proportional to the square of their radii. Place a candle in the center of a sphere of given radius, and each part of this sphere will receive a certain quantity of light. Double the radius of the sphere, and as the same quantity of light is spread over a surface four times greater than before, its intensity over the same extent of the sphere will be four times less; while with treble the radius, the intensity will be 9 times less, and so on. It would be the same if the source of light were replaced by a sonorous body. The intensity of sound per unit of surface would be, for the same reason, inversely as the square of the distance. This law of inverse squares simply signifies therefore that the intensity at a given distance is inversely proportional to the surface of the spherical wave propagated to that distance, which is geometrically evident. When a force decreases with the distance in accordance with this law, it is legitimate enough to imagine that it is propagated by spherical waves. This should be the case with gravity.
2. The Consequences of Gravitation ~
The laws of gravitation simply express this experimental fact, that all bodies exercise a certain attraction upon one another. From this phenomenon, apparently simple, although inexplicable, results the course of the stars, and, according to some physicists, all the forces of the universe, including those which governed the formation of our solar system.
It is, in fact, generally admitted that the central globe which was the origin of our own, must have been formed by the reciprocal attractions of the elements of the primal nebula.
The maintenance of the heat of the sun, whence is derived the greater part of terrestrial forces, must be the result, according to Helmholtz, of the progressive contraction of the sun following on the attraction of his elements for each other and the loss of vis viva [kinetic energy] which the molecules experience when coming close to each other.
It is likewise by gravitation combining its effects with those of inertia that the planets describe their orbits in space. A body launched in a straight line would continue its course I the same line by reason of its inertia, but if it be subjected at the same time to the action of a force which attracts it in a direction perpendicular to that of its course, its rectilinear trajectory is deflected and becomes a curve. Their inertia acting by itself would have compelled the planets when they detached themselves from the sun at the moment of their formation to continue their course in a straight line through space. On the other hand, had they not received this initial impulse, gravitation would have brought them together into one single mass. The result of these two antagonistic actions is the curve described by them round the sun, which can, according to the extent of the impulse received in the first instance, be a circle, an ellipse, parabola, or hyperbola. Certain comets alone seems to have a hyperbolic trajectory, for the planets follow ellipses which are almost circles. All the planets of course act upon one another, which is why Kepler’s laws are only approximately exact.
Centrifugal force, so named, results from this double action of the original impulse combined with gravitation. The star represents the stone held by the cord whirled by the slinger. The impulse received by the stone represents that received by the star, and the cord which keeps back the stone corresponds to attraction. This attraction is the immaterial string which attracts the star without ceasing, and compels it to treads a circular path. If it ceased to act for a single instant, the planets would escape into space in a straight line by following the tangent to their trajectory, like the stone which the cord fails to hold during its rotation.
Thus, then, the movement of the stars is the result of two causes: a permanent force (gravitation) which acts on them without ceasing, and an inertial impulse which has given to the star a certain velocity which it keeps by reason of the principle of inertia, whereby a body set in motion continues in motion. We are, then, thrown back on the mysterious property of inertia studied above, which is perhaps more incomprehensible than gravitation.
Gravitation, which is the origin of the forces and of the movements of the stars, is also that of the phenomenon called weight. This last is only a particular case if universal attraction, i.e., of the attraction which bodies exercise upon each other. When we say that a body possesses a given weight, it means that it is attracted by the earth. We measure the magnitude of this attraction by inquiring the pressure which it exercises on another body, the scale-pan of a balance for instance, or by opposing to it an antagonistic force of known magnitude, such as the elasticity of a spring or the attraction of a magnet.
We most often measure and utilize forces by putting them in opposition to weight. The greater part of our machines are constructed either to use weight or to strive against it. Industrial mechanics is in reality the art of utilizing weight and inertia.
Gravitation, inertia, and solar heat represent the three fundamental power utilized by man. If these magnitudes had been different, civilization would have taken a different aspect. So far as solar heat is concerned, we see this clearly when we notice how different are the fauna and flora at the Pole and at the Equator. Extreme heat and extreme cold alike seem to imply savage or at least barbarian life. Below 0° C and above 50° C, no civilization can be born.
We perhaps see less clearly the phenomena which differences of gravitation might generate. It is easy, however, to anticipate how different would be the conditions of our existence if weight were reduced or assimilated. At first sight, we seen to have no right to imagine its non-existence, since it is, like inertia, an irreducible property of matter incapable of modification, and following bodies through all their changes. But, if gravitation remains indestructible, its effects might be restricted or reduced to nothing. It has been calculated that it would suffice for the earth to turn 17 times as fast as it does now for the centrifugal forces to entirely annul the attraction of the earth’s mass on bodies at the Equator. They would then cease to have weight, and consequently would not fall back on the earth when left to themselves. Their weight therefore depends on the speed of rotation of our globe, which itself depends upon the impulse received in the first instance, when, under the influence of centrifugal force, it detached itself from the sun at the time of its formation.
There are other causes possible by which attraction might be annulled. It will be remembered that when the waves of light fall upon a source, they exercise a certain pressure upon it. It has been calculated that for portions of matter of small density and finely divided, the attraction of the sun might not only be annulled but even changed into a repulsion, which may be the cause of the deformation of the heads of comets. Poynting has calculated that a sun a the temperature of our own, it had only 32 km diameter, would repel, instead of attracting, all bodies less than 1 cm through.
It would be useless to dwell longer on these considerations, which show us merely how numerous are the possibilities of things, and how much the existence of living beings and all the ideas which we form of the world are conditioned by external forces from which we cannot withdraw ourselves. This is a self-evident notion, but one which certain philosophers rather forget.
3. Forces Dimly Seen ~
It is hardly to be imagined that the forces of nature are limited to the small number of those with which we are acquainted. If we are ignorant of them, it is because we have no reagents which disclose them. The discovery of appropriate reagents is the sole means of putting them in evidence. During the last 20 years, science has annexed the Hertzian waves, the x-rays, the cathode rays, the radioactive rays, and intra-atomic energy to the small kingdom of the forces known of old. It is difficult to believe that the end of these discoveries is reached, and mighty forces may surround us without our knowing it. Electricity, unknown for thousands of years, would perhaps still remain so if all bodies were good conductors.
This is no pace evidently for a dissertation on things of which we are ignorant. One ought first to try to discover them. A few hints hardly allow us to suspect their existence.
Our means of research are generally the verification of attractions and repulsions, and do not take us very far. Several times, however, the attractions and repulsion which a light needle suspended by a thread of raw silk experiences when approached by a living body has been pointed out; but they are perhaps due merely to the action of heat. (1).
[(1) Mr Legge tells me that he has established the fact that those actions do not manifest themselves through a vacuum. I made some experiments with this some years ago, and gave in an article in a popular magazine, Pall Mall Magazine, April 1902, a description of an apparatus which I believe to be very much more sensitive than that referred to in the text. Recently, however, it occurred to me to surround the apparatus with what is known as a vacuum jacket, to invert over it a double bell glass, having a vacuum between its outer and inner walls. With this, the needle remains motionless, no matter what bodies are brought near to the outer cover of the apparatus. As a vacuum cuts off not only electrical influences but also heat rays, it seems most probable that the motion of the needle is, in the cases originally quoted, the result of convection current formed by heat in the interior of the receptacle. -- Ed.]
We are, then, here entirely within the domain of pure hypothesis, and we ought not to stay there long. Let us merely say that intra-atomic energies are a source of many possible varieties of energy. Mr Georges Delbruck, an engineer, has suggested that the larger birds, whose soaring flight without apparent motion is so difficult to explain, may have the faculty of generating at the expense of intra-atomic energy a force capable of striving against gravitation until it renders it null. This hypothesis is very difficult to verify, but it is not fundamentally absurd. Gravitation is only an attraction, which can be annihilated by a corresponding repulsion, as that exercised on masses of iron can be annulled by a magnet.
This theory will delight the spiritualist, who believe that they have verified the levitation phenomena which objects in relation with certain persons seem to present. Nevertheless, before explaining these levitations, their reality must first be rendered beyond dispute.
As to the so-called psychic forces, materializations, etc., it will be useless to busy ourselves with them here. They have attracted attention of eminent scholars, such as Crookes, Lodge, Richet, and others, but they have yet to be demonstrated, and until this is done, it is better to try to interpret the phenomena observed by known causes. I had occasion to examine without prejudice, and with the assistance of M. Dastre, a subject, with a European reputation, but our investigations, continued throughout several séances, disclosed to us nothing demonstrative. The story of the N-rays, moreover, shows us the difficulty of thorough observations in similar matters, and of avoiding causes of error. Before building a temple to unknown forces, we ought to be perfectly certain that they do not issue wholly from that domain of illusion in which all divinities have hitherto been born.
The Molecular and Intra-Atomic Forces
1. The Attractions and Repulsions of the Elements of Matter ~
Several chapters of The Evolution of Matter were devoted to the study of the equilibria of the elements of matter, and the forces of which they are the seat. We will run through them again to show how...
[ Pages 353-354 Missing ]
action is apparent. We call lines of force the directions in which the attractive and repellent effects are produced. While very easy to observe in electrical phenomena, the molecular fields of force due to other actions, may be made equally evident by different artifices, as shown in The Evolution of Matter.
The most important of the molecular forces is that called cohesion. The existence of the bodies of which the universe is formed is due to its influence. It conditions their forms. Without its action the word would vanish in an invisible dust of ether.
Although cohesion is an extremely powerful force, as is proved by the huge mechanical effort necessary to separate the particles of bodies, we possess in heat the means of annulling it.
As soon as the molecules are sufficiently separated by heat, the most rigid body loses its consistence and becomes liquid or gaseous. The very fact that there is no cohesion as soon as the molecules are apart from each other, proves that the molecular attractions which produce cohesion only act at a very small distance. Bodies in powder, even when strongly compressed, do not become hard for this reason. However near to each other these particles may be brought by pressure, they are still not enough to attract one another. To bring them near enough for this purpose demands an enormous pressure. Spring was thus able to form blocks of brass by energetically compressing powdered copper mixed with powdered tin.
Simple cooling produces the same effect, which is why, when we allow a melted body to cool, it returns to the solid state. Temperature constitutes one of those specially appropriate reagents of which we have so often taken occasion to show the importance. To separate the molecules kept together by cohesion demands an enormous effort, while only a relatively feeble one is necessary to separate them by heat.
Nothing resists the molecular forces. We can split a bombshell full of water by freezing it. We have only to touch a body for its molecules to separate a little.
Cohesion, which is already much reduced in liquefied bodies, disappears altogether in gaseous ones. Far from attracting each other, the molecules of gases behave -- at least in appearance -- as if they repelled each other. Thus, the smallest quantity of gas, introduced into a receiver, spreads into all parts of it. To compress a gas -- to force its molecules to draw together -- a considerable pressure must be exercised upon it.’
In solution, the molecules of the dissolved bodies behave in a similar way. They exercise upon the walls of the vessel containing them a pressure called osmotic, which is easily measured. The solution of a body has for this reason been compared to a gas.
In the energy of cohesion we have therefore a force which is very great, but which has the characteristic of only acting upon the particles of bodies when they are a very small distance apart. This peculiarity is not found in the attractions which constitute gravitation. This acts on all bodies at all distances.
We can form a summary idea of the forces which may produce cohesion by creating something similar in a body -- that is to say, a force capable of generating attraction. If we cause an electric current to circulate in a metal wire surround an iron rod, we give to the molecules of the rod a power of attraction sufficient for them to exercise upon a piece of steel an attraction which may rise as high as 100 kg/sq cm. In the field of force surrounding the metal, a considerable attraction may also be exercised. One thus has a glimpse of the way in which the ether can assume a form making it capable of exercising extremely energetic attractions.
It has just been said that molecular attraction and repulsions are only manifest at a slight distance. There are, however, some which act from fairly far off. A piece of charcoal cooled to -200° C will so energetically absorb the air of the receptacle in which it is placed as to make a vacuum in it. A hydrometric body acts in the same way in respect of the water vapor surrounding it. Tantalum, heated to 600° C in an atmosphere of hydrogen, absorbs 600 times its volume of the gas in which it is plunged.
2. The Molecular Equilibria ~
All the attraction and repulsions just spoken of are in equilibrium with each other, and also with the external forces surrounding them. Matter, which was formerly conceived to be independent of its surrounding medium, represents only a state of equilibrium between the external forces of which it is the seat and the external forces which envelop it. The most rigid body is transformed into vapor as soon as this medium is sufficiently changed. In fact, we cannot define any property of a body -- inertia excepted -- without mentioning the conditions of the medium in which it is plunged. Even when we do not see their variation, the properties of a body follow the least changes of its medium to put themselves in equilibrium with it. It suffices to bring the hand near matter for its equilibria to be modified. A variation of one-millionth of a degree in the medium in which it is plunged varies the electrical resistance of a body in a way detectable by our instruments. The bolometer is based on this principle.
If we wish to point out the physical properties of any substance -- water, for instance -- we must always for completeness give the conditions of the medium. Two different saline solutions with the same osmotic pressure have the same freezing point; while the vapor-pressures of liquids are equal at temperatures equally removed from their boiling point, and bodies of the same atomic weight possess the same caloric capacity, etc.
Van der Waals has gone further still in his law of corresponding states, which shows that if the volume, pressure and temperature are estimated by taking as units the initial quantities, one equation is enough to translate the properties of all fluids. This means that all have the same properties in corresponding states (1).
[(1) The generality of the law of corresponding states is here rather exaggerated. It seems to be true for certain derivatives of benzene only. See Poincare, The Evolution of Modern Physics, p. 113)
3. The Force and the Form ~
By molecular attractions and repulsions we can go very neat to an explanation of certain phenomena, but our ignorance in regard to the causes which give matter its form is complete. Why, for instance, do liquids when solidifying take certain regular geometrical forms which are invariable for each body? The hidden causes of the form of a crystal are as unknown to us as those of a plant or an animal. These things happen as if physico-chemical phenomena were governed by unknown forces, which compel them to act in a predetermined direction.
We may, however, in some sort comprehend the genesis of a few forms by reducing them to extremely simple cases -- for instance, the molecular attractions within a liquid. When we introduce into an aqueous solution a drop of liquid of different osmotic pressure, the molecules of the two liquids are attracted or repelled, and sometimes form fairly regular figures. We may also, by combining attractions and repulsions of electrical origin, obtain greatly varied figures. Some have been given in The Evolution of Matter, which were formed by combining the attractions and repulsions of the particles of dissociated matter.
By similar means we may obtain figures imitating plants. For the last 20 years certain observers, like Traube, have exercised their ingenuity in creating such reproductions. Of these, M. Stephane Leduc has obtained the most curious results, as may be judged by the figures given here. The mode of production indicated by him is very simple: "A granule of copper sulfate 1 mm to 2 mm diameter, composed of two parts saccharose, 1 of copper sulfate, and water to cause it to granulate, is planted in an aqueous solution containing 2% to 4% of potassium ferricyanide, 1% to 10% sodium chloride and other salts, and 1% to 4% of gelatine. It germinates in a space of time which varies from a few hours to a few days according to the temperature. The granule surrounds itself with a membrane of copper ferricyanide permeable to water and certain ions, but impermeable to the sugar enclosed within it, which produces in this artificial seed the strong osmotic pressure which determines its absorption and growth. If the liquid is spread over a glass plate, the growth takes place on the horizontal place; if the culture is made in a deep basin, it takes place at once horizontally and vertically. One single artificial seed may give 15 to 20 stalks sometimes as high as 25 cm".
Figure 41 ~
Figure 42 ~
We may think we see here the image of life, but there is hardly any more connection between these artificial plants and real ones than there is between a living man and his statue. Their production merely shows that osmotic equilibria may condition certain external forms.
We have studied in this chapter only the relatively simple forces -- primary forces, we might say -- that govern the mineral world. They are, however, very obscure. When we come to the forces which direct the phenomena of life, the obscurity becomes greater yet.
The Forces Manifested by Living Beings
1. Living Matter and Cellular Life ~
We observe among living beings very distinct manifestations of energy: (1) physico-chemical forces, such as heat, light, electricity, described in physics; and (2) those united under the name of vital forces, of the nature and mode of action of which we are profoundly ignorant. For the sake of completeness I am obliged to speak of these last, but I do so with the certainty that I can say nothing useful on the subject. To descant on the phenomena of life while we are incapable of explaining why the stone which leaves the hand falls to the ground is a task which must be left to the leisure of metaphysicians.
The sole interest which this chapter may possess lies in its showing exactly the present limits of our slight acquaintance with those still very incomprehensible phenomena, the synthesis of which constitutes life. All living beings without exception are composed of an aggregate of little microscopic elements called cells. These, although if such an extreme minuteness that their diameter hardly exceeds some thousandths of a millimeter, have yet an extremely complicated structure, and properties even more so.
In a very summary way we may say that they are composed of a granulous substance called protoplasm, containing in its midst a nucleus of filaments. The microscope has shown the structure of this dust of life, but the marvelous part is that which we do not see, It contains, in fact, the germ of ancestral forms and those which will be born in the future course of its evolution. Every living being is derived from one primitive cell -- the ovum, the transformations of which suffice to form a complete being in a very short space of time.
These cells, then, constitute the materials from which living beings are built. The lowest of these beings are composed of a single cell. They multiply, stick together, and are associated with the higher ones to form their different organs. The higher being is therefore a veritable society of cells having each its destined function, but working together in the general interest. The higher animal possesses a nervous system which puts the organs formed by the cells, and the digestive and circulatory apparatus which brings to them the elements of nourishment, in relation with one another, while the other organs are charged with the expulsion from the system of the remains of their nourishment, and so on. The whole living being labors to maintain the cells; they depend on it, and it on them.
The protoplasm of which the cells are composed represents the stock of life common to all living things, from the most humble to the highest. It fulfills universal functions, assimilation as well as destruction, and, from the chemical, anatomical, and physiological standpoints, seems to be little modified from one being to another, while its forms change to an infinite extent. Nature, which economizes her efforts, has need of little in order to vary the elementary structure of beings.
It is by the study of the life of cells that we may best understand the profundity of the mystery of life and its amazing complexity. To show this it will be enough to recall what appears on this subject in The Evolution of Matter.
The chemical studies which humble cells know how to manufacture comprise not only the most skillful operations of our laboratories, such as etherification, oxidation, reduction, polymerization, and so on, but many others demanding still more skill which we cannot imitate. By means which we do not even suspect, the vital cells construct their complicated and varied compounds -- the albuminoids, cellulose, fats, starch, etc., necessary to the support of life. They are able to decompose the most stable bodies, such as sodium chloride, to extract the nitrogen from the ammoniacal salts, and the phosphorus from the phosphates, etc.
All these labors, so exact and so admirably adopted towards one end, are directed by forces of which we have no idea, and which behave exactly as if they possessed a second sigh far superior to our reason. That which they are accomplishing every instant of our existence soars far above all that the most advanced science can realize. The scholar capable of solving by his intelligence the problems solved every moment by the cells of the lowest creature would be so much higher than other men that he might be considered by them as a god.
Although protoplasm is the seat of an incessant activity, it is only slowly renewed in each being, and does not in fact contribute to its support out of its own substance, but by the materials it incorporates. It hardly uses itself up more than an engine fed by coal.
2. Instability is the Condition of Life -- Part Played by the Intra-Atomic Energies ~
Life is only maintained by an incessant using up of the materials borrowed from outside. The cell assimilates and destroys without pause. Instability is the law of life. As soon as it is succeeded by stability there comes death.
Life is, then, the result of a constant exchange between the living being and the medium in which it is plunged, A being cannot be isolated from this medium, and would even be incomprehensible without it. It is the differential action of assimilation and destruction which governs the ascending or descending evolution of beings throughout their existence. Te cell dies without ceasing, but as it is renewed without ceasing, the living being has an apparent stability. It resembles a building the stones of which are every day removed by cunning demons, but are immediately replaced by other demons. The building does not change its appearance, and only begins to grow old when the restoring demons incompletely perform their task. The day when they no longer fulfill it, the building falls in ruins.
The maintenance of life represents a great expenditure of the energy furnished by food. With a great number of animals this last is constituted of vegetables, which by themselves can raise mineral matter to the degree of complexity necessary for the creation of chemical compounds charged with a slight volume of unstable energy. Thanks to them, matter is unceasingly raised from the unorganized state, to return at length to the starting-point.
But if the instability of the chemical elements of the cells is the very condition of life, does not the instability of the atom also play a part in these phenomena? I would not seem to exaggerate the influence of that intra-atomic energy which I have already made use of to explain many phenomena, but one must believe that its possible part in vital action is often present to the mind, for I have received letters from many doctors on this subject. I should not, however, have spoken of this, if experimental facts did not seem to demonstrate that the dissociation of matter may be manifested within the organism. Thus, for instance, Prof. Dufour has recently shown that air that has been breathed contains radioactive particles. Now these particles result, it will be remembered, from the dissociation of matter. I am also led to believe -- and M. Dastre, to whom I have submitted my hypotheses, entirely admits the fact -- that we must seek in atomic dissociations for the origin of the increase of energy produced by certain excitants, such as cola, caffeine, etc., whose composition clearly shows that they cannot be considered as foods. Some diastasic actions may well have a similar origin. The elements of protoplasm are considered at the present day as very unstable colloidal substances, and I have shown that these substances often come from the dissociation of matter. Given the colossal energy of intra-atomic energy. We can understand that the cell may become a mighty generator of energy without its composition being perceptibly altered [Biological transmutation. -- Ed.]. It must also be remarked that physiologists have never succeeded in furnishing an acceptable theory of the action of the excitants spoken of above. I hope that what has been said will permit them to give some explanation of these phenomena. These intra-atomic energies, set free within the organism, seem, moreover, of rather exceptional occurrence, and only intervene under the influence of certain excitants, when the living being is obliges to make a considerable effort rapidly. In normal conditions the forces which it manifests have especially as their origin the chemical energies which come from foods.
These last may be very varied, but may be reduced, it will be remembered, into three categories:
(1) Albuminoids (egg white, animal flesh, etc.)
(2) Carbohydrates (starch, sugar, etc)
(3) Fats
We must add to these the oxygen of the air absorbed by respiration and necessary to displace, by combining with them, the energies of the chemical compounds introduced into the organism.
The food value of alimentary substances is sometimes estimated by burning them in a calorimeter and measuring the calories they produced. This rather barbarous process would lead us to consider coal and petroleum as valuable foods, as they disengage by their combustion many calories. One kilogram of coal generates about 8000 calories, while the adult uses only about 2500 a day. Prof. Chauveau has said: "We must give up looking for the nutritive value of food in their heat of combustion. The theory of food and alimentation cannot be presented under so simple a form".
All foods produce heat, but this being the last term of the energetic changes of the organism, and not the first, it is evident that it is not this which generates the vital forces. Its part seems to be merely to put the elements of the organism in the conditions necessary for their proper working. M. Dastre made this point evident some time ago.
When all the foods have given up their energy to the organism, they are cast forth under different forms, such as water, carbonic acid, urea, etc., deprived of usable energy.
To sum up, we see that the vital forces are maintained by the chemical forces derived from the food, and that it is these last which uphold the first.
3. The Forces Which Regulate the Organism ~
Even when we liken to physico-chemical forces the vital forces manifested by living beings, it must be recognized that some things happen as if there existed quite peculiar forces, some of which are intended to regulate the functions of the organs, and others to direct their forces. They must be described, for the sake of clearness, by a name, although they probably are only a synthesis of actions which are very different and not yet dissociated. We will call the first category regulating and the second morphogenic (i.e, generators of forms) forces.
In spite of the efforts of thousands of workers, physiology has been able to tell us nothing of the nature of those forces. They have no analogy with those which are studied in physics.
The regulating forces act as if they watched over the proper working of the living machine, regulating the temperature and the constancy of the composition of the blood and other secretions, limiting the oscillation of the different functions, adapting the organism to the changes of the outer world, etc. They hold undivided sway over that region of unconscious life which constitutes the greatly preponderant part of the existence of beings. The philosopher may deny their existence, but the physiologist, who sees them perpetually in action, hardly contests it. He generally recognizes, like Claude Bernard, true "directing principles which direct phenomena which they do not produce and physical agents producing phenomena which they do not direct".
These real or apparent directive actions formerly caused us to admit the existence of immaterial agents, the soul or vital principle, independent of the body and capable of surviving it. In reality it is not one single directive soul that we should have to imagine, but many souls, for the life of an individual appears to us as the sum of small cellular lives almost infinite in number, and all fulfilling very different functions. Thanks to these directive forces, nature shuts up each organ in the sphere designed by her, and constantly brings them back to it with the two great springs of all the activity of beings -- pleasure and pain.
The regulating forces present the peculiar characteristics of being conditioned by a long ancestral past and of modifying it in every generation. The most modest cell, an amoeba or a globule of protoplasm, is charged with a past. To this must be attributed the variety of the reactions of living beings under the influence of external agents. In any vital act, and psychical manifestations must be included among them, our ancestors act as well as ourselves, but as their number is immense, there exists in every being the possibilities of action dependent on excitants capable of calling them forth. Many ancestors speak in us, but according to external circumstances they are not all equally heard. It is exactly this enormous past with which the least cell is saturated which makes so illusory our attempts to create living matter. "To create living matter!", writes M. Gustave Bonnier. "How can it be hoped for in an instant in the present state of science, when we think of how many accumulated characteristics, how much heredity, how much complicated future, there are in a fragment of living protoplasm? If we think that the development of a higher animal, its successive transformation in the embryo into a protozoan, into a worm, into a fish with gills, finally producing a mammal, a man -- and that all these future forms are found potentially in a microscopic fragment of inchoate living substance! If we reflect that this reminiscence of distant ancestors, this heredity acquired during tens of thousands of centuries all exist in this minute drop of protoplasm, we then understand the meaning of the truth, that it is not more difficult to create afresh a living animal -- an elephant for instance -- than to create a speck of living matter. When man shall solve this last problem, he will have become more creative than the Creator, stronger than all Nature, mightier than the Universe itself!"
4. The Morphogenic Forces ~
The acquisition of a specific form belongs as well to minerals as to living beings, since mineral can assume the geometric contours characteristic of each. We have already studied their genesis when speaking in The Evolution of Matter of the life of crystals.
But these substances of the mineral world only represent a very low order of life, fixed to some extent in invariability of form, and there is only a distant analogy between the life of an animal which constantly assimilates and destroys, and the immobility of a crystal. It does not represent a living form of matter, but only the ultimate term of life.
The medium supplies to the living being the matter of which it is composed, the energies which it expends, and the excitants which put it into play. All these external conditions may modify its form, but they act on a basis of life that they cannot create. Until now only life has been able to create life. That it may have been born spontaneously at the dawn of the geological ages under the influence of unknown reactions is very probable, for it must needs be that life has a beginning, but we cannot say why it began, and we do not know how to make it begin again.
The living being alone, then, enjoys the property of generating a substance analogous to its own and possessing the same forms. Every cell, and even every organ, possesses the mysterious power of creating forms similar to itself. Among the lower animals. Very part of the animal when but in pieces reproduces the wounded part. Among animals of fairly high organization, such as the tritons and the salamanders, a limb cut off, an eye torn out, is soon reborn.
By going back to the real origin of each living being -- i.e., to the cell from which it is derived -- we see better the inexplicable side of these morphogenic forces. This primal cell will undergo, tanks to them, the series of transformation which lead it to form a tree, a bird, a whale, or a man. It contains then, potentially, all the forms which go forth from it, and which always evolve in the same way for each species.
Such forces are, as M. Dastre has justly observed, "the most refractory and the most out of the reach of physico-chemcial explanations. How shall we explain", he says, "this unfathomable mystery which makes the egg cell, drawing to itself materials from without, progressively build up the astonishing construction which is the body of an animal or of man himself?".
All our attempts at interpretation of such a phenomenon are so perfectly futile, that it is better to give them up than to formulate them. The eminent physiologist last quoted points this out: "The naturalists give us nothing but words. They speak of heredity, of adaptation, of atavism, as if they were real and efficient beings, while they are only appellatives and names which are applied to collections of facts".
These terms of adaptation, heredity, etc., are, however, not entirely vain words if we use them to simply translate facts of observation instead of considering them as explanations. They mean simply that forces utterly unknown, and of the nature of which we can have no suspicion, oblige the primal cell of a being to become an animal or a plant, and to bequeath to the beings derived from it the changes to which it has been subjected by different actions, such as that of the medium. As the cell possesses such powers, we conceive that, in the immense ages of the past, forms adapted to all the variations of the medium may have succeeded each other. It would seem that with a common stock of life, a sort of clay of beings, nature has tried numberless combinations, of which some only have been able to survive. We meet at the present day in geological debris some which seem the fancies of artists haunted by the visions of demoniacs. Such are, for instance, the gigantic Diplodocus, the Brontosaurus, and others, true dream monsters.
All these varied efflorescences of life formerly appeared to materialists as the fancies of a Creative Power drawing them forth periodically from the chaos which preceded the expression of his will. The immense service which Darwin rendered to the scientific mind was to eliminate the supernatural causes of the concatenation of things by making us see that laws which know no caprice, such as adaptation, selection, and the survival of the fittest, can explain the transformation of living beings. The theory of evolution is tending to be in part replaced at the present by that of abrupt mutations, the existence of which among certain vegetables has been disclosed by patient observations. But by showing us the possibility of finding scientific explanations in a domain where they seemed to have no place, Darwin changed the orientation of ideas in the most important branches of scientific activity. During half a century the learned world has been inspired by the doctrines of this mighty genius. One may say of the Origin of Species what Sainte-Beuve formerly wrote of the Esprit des Lois: "There is no book which we can quote as having done so much for the human race at the time when it appeared, and from which a reader of our day can draw so few applicable and positive ideas. But this is the destiny of nearly every work which has caused the mind of man to progress".
The theory of abrupt mutations, which has shaken some fundamental facts of Darwin’s doctrines, is still in its infancy. The mutations which have been observed are rather rare, and do not bear upon fundamental characteristics. We have been able, however, to make use of them to obtain new species of cereals, the characteristics of which are reproduced by heredity with regularity and constancy. The scientific and philosophic importance of these mutations especially lies in the establishment of the notion that certain changes, prepared doubtless by an invisible and previous evolution, may take rise abruptly.
This fact will perhaps explain to us why at certain geological epochs there have suddenly appeared a whole series of living species so different from those which went before and those which come after them, as to make it very difficult to establish a link between them. The lacunae which science has vainly sought to fill should then, as Cuvier thought, be very real.
We assuredly observe in the succession of beings a certain continuity, a general direction, but not that regular and lineal continuity which many naturalists still imagine. If the evolution of beings is represented by a curve, this curve should indeed have a general regular trajectory, but would contain many solutions of continuity and loops.
The evolution of beings seems to have been obtained only at the price of repeated essays which seem useless enough to the human eye. From the point of view of our limited intelligence, we might say that things happen in nature as if they were sometimes directed by superior intelligences, and sometimes by absurd combinations due to blind grouping of improbable chances. Nature seems to be at the same time full of foresight and of blindness. It is very possible, however, that both the foresight and blindness are only in our minds. She has no doubt as guide and as means of action elements which we do not suspect, and we cannot in consequence judge her. We must always distrust hypotheses made on the subject of a domain which no human eye has ever seen.
Nor is there any occasion to bring into our explanation of things supreme beings with whom we are not acquainted. It was by eliminating them that science effected her greatest progress. We have seen that simple cells realize in the organism chemical operations beyond the resources of our laboratories, and act as if they were unceasingly guided by a superior intelligence. No one imagines, however, that there is such an intelligence behind each cell, and there is no occasion to imagine it for the aggregate of cells which constitute any animal whatever. Their structure disclosed an extremely skillful mechanism directed by forces of which at the present day we do not know the nature, but which we may hope to one day bring within the cycle of our knowledge.
5. The Interpretations o the Phenomena of Life ~
What has been said shows that the phenomena of life were always the stumbling block of philosophy and physiology. "Physiology", writes M. Dastre, "cannot answer the question of the ages -- What is life?". The philosophers have not been able to answer it either, or, at least, none of their answers could bear criticism. "Philosophy offers us", writes Dastre, "in order to conceive life and death, hypotheses. It offered us the same 30, 100, 2000 years ago: animism, vitalism under its two forms, or the doctrine of vital properties, and finally materialism, mechanism, or unicism, or monism (to give it all its names), that is to say, the physico-chemical doctrine of life".
It is this last explanation which predominates at the present day. It is not, perhaps, the most certain, but it is certainly the most fertile, since it excites researches which render useless the vitalistic and animistic theories which endow living beings with an incomprehensible power -- the soul or vital principle -- the power of which dispenses us from seeking further for explanations.
The true problem which now presents itself for solution, and will doubtless do so for a long time yet, is this: Have vital and psychic manifestations distinct causes? Are the vital forces absolutely different from those with which we are acquainted? Do they represent an independent and irreducible category? Is it possible to pass from the laws of crude matte to those of living matter?
Up till now we have found no bridge capable of linking together these two orders of phenomena, and the gulf which separates them appears yet deeper if among the vital forces we include the psychical phenomena which end in intelligence.
This link which we do not see will perhaps some day appear, and we may already guess how it will be found. As continuity seems a general law of nature, it is not in the higher being that the vital and psychical phenomena must be studied, because there their complication makes them too inexplicable. But by descending to the very first stages of life we shall discover the outline of an explanation of psychical phenomena. Among quite primitive beings, such as a globule of protoplasm, we observe, as several volumes of researches have shown, acts well adapted to the end to be achieved and varying according to the circumstances, as if the being were capable of rudimentary reason. But these are perhaps only physico-chemical reactions generated by external excitants. Their interpretation by the hypothesis of simple reaction is very insufficient. We are evidently confronted by a chain of causalities of which we possess no single link, and which consequently we do not understand. We shall not penetrate any further into them by imagining, to explain them, immaterial principles endowed with the attributes we ourselves lend them. This would be to return to the time when the will of Jupiter produced the thunder and that of Ceres the crops.
The learned ought to shun supernatural explanations, and also those which are too simple. The materialist and spiritualist interpretations are only words devoid of all worth and only of interest to those minds which prefer any explanation to none at all.
We must seek, and not invent explanations. The book of nature is one which takes long to read. The few pages painfully deciphered after centuries of effort show us a universe much more complicated than it appeared at first. Our sciences are built up on concepts representing merely interpretations capable of adapting themselves to the little fragments of things which are within the reach of our intelligence.
If it be true that we do not know the causes of life, nor even the final reason of a single phenomenon, nothing warrants our saying that we shall always be ignorant of them, We confine ourselves within a barren philosophy when we declare unknowable that which is only unknown as yet. Science descends a little further each day into the mysterious gulfs where the last elements of things lie hid. But as a philosopher has rightly said, our sounding-rod is still too short for the immensity of such abysses.
Top ~ Home ~ Catalog ~ Links
rexresearch.com